Understanding how many valence electrons carbon has is crucial for grasping its chemical behavior. At HOW.EDU.VN, we provide expert explanations to simplify complex scientific concepts. Carbon has four valence electrons, enabling it to form stable bonds with a variety of other elements. Dive deeper to understand electron configuration and its impact on chemical bonding, Lewis structures, and the octet rule.
Search Intent Keywords: valence electrons, electron configuration, chemical bonds, carbon atom, Lewis structure
1. What are Valence Electrons?
Valence electrons are the electrons located in the outermost shell, or valence shell, of an atom. These electrons determine how an atom will interact with other atoms to form chemical bonds. The valence of an element is the number of electrons it needs to gain, lose, or share to achieve a stable electron configuration, typically resembling that of a noble gas. Understanding valence electrons is fundamental to predicting chemical properties and bonding behavior.
1.1 Defining Valence and Valence Electrons
Valence indicates the number of electrons an atom requires to either gain or lose to attain the electron configuration of the nearest noble gas. Valence electrons, on the other hand, are the electrons present in the outermost shell of an atom, defining its stability and influencing its interactions with other atoms during chemical bond formation. These electrons are pivotal in determining an atom’s chemical properties.
1.2 Significance of Valence Electrons
The number of valence electrons dictates an atom’s chemical properties, influencing whether it will form ionic bonds, covalent bonds, or metallic bonds. Atoms with full valence shells, like noble gases, are stable and less reactive. Atoms with incomplete valence shells tend to form bonds to achieve stability. This drive to achieve a full valence shell is a primary driver of chemical reactions.
1.3 Valence Electrons and Chemical Bonding
Valence electrons are directly involved in chemical bonding. They determine the type and number of bonds an atom can form. For example, elements with one valence electron, such as alkali metals, readily lose that electron to form positive ions, while elements with seven valence electrons, such as halogens, readily gain an electron to form negative ions.
2. Understanding Carbon’s Electron Configuration
Carbon’s electron configuration is key to understanding its unique bonding capabilities. Carbon has an atomic number of 6, meaning it has 6 electrons. These electrons are arranged in specific energy levels or shells around the nucleus. The electron configuration of carbon is 1s²2s²2p².
2.1 Electron Configuration Explained
The electron configuration 1s²2s²2p² indicates that carbon has two electrons in the first energy level (1s orbital) and four electrons in the second energy level (2s and 2p orbitals). The first energy level can hold a maximum of two electrons, while the second energy level can hold up to eight electrons.
2.2 The Role of Orbitals
Electrons reside in specific regions around the nucleus called orbitals. The s orbitals are spherical, while the p orbitals are dumbbell-shaped. In carbon, the two electrons in the 1s orbital are closest to the nucleus, followed by the two electrons in the 2s orbital and two electrons in the 2p orbitals.
2.3 Carbon’s Valence Shell
Carbon’s valence shell is the second energy level, which contains four electrons (2s²2p²). Since the second energy level can hold up to eight electrons, carbon needs four more electrons to achieve a stable, full valence shell. This requirement is why carbon forms four covalent bonds.
3. How Many Valence Electrons Does Carbon Have?
Carbon has four valence electrons. This is determined by its electron configuration, specifically the number of electrons in its outermost shell. These four valence electrons enable carbon to form a wide array of compounds with diverse properties.
3.1 Determining Valence Electrons from Electron Configuration
By examining carbon’s electron configuration (1s²2s²2p²), it is clear that the outermost shell (n=2) contains four electrons (2s²2p²). These four electrons are the valence electrons and are responsible for carbon’s bonding behavior.
3.2 Carbon’s Position in the Periodic Table
Carbon is located in Group 14 (also known as Group IVA) of the periodic table. Elements in Group 14 have four valence electrons. The periodic table provides a quick way to determine the number of valence electrons for main group elements.
3.3 Implications of Having Four Valence Electrons
Having four valence electrons allows carbon to form four covalent bonds. This tetravalency is the basis for the vast field of organic chemistry, where carbon forms chains, rings, and complex structures with other elements like hydrogen, oxygen, and nitrogen.
4. Carbon and Covalent Bonding
Carbon’s four valence electrons enable it to form four covalent bonds. Covalent bonds involve the sharing of electrons between atoms. Carbon typically shares its four valence electrons with other atoms to achieve a stable octet, meaning eight electrons in its valence shell.
4.1 Understanding Covalent Bonds
In a covalent bond, atoms share one or more pairs of electrons to achieve a stable electron configuration. Carbon can form single, double, or triple bonds, depending on the number of electron pairs it shares with other atoms.
4.2 Single, Double, and Triple Bonds
- Single Bond: Carbon shares one pair of electrons with another atom (e.g., in methane, CH₄, carbon forms four single bonds with four hydrogen atoms).
- Double Bond: Carbon shares two pairs of electrons with another atom (e.g., in ethene, C₂H₄, each carbon atom forms a double bond with the other carbon atom and two single bonds with two hydrogen atoms).
- Triple Bond: Carbon shares three pairs of electrons with another atom (e.g., in ethyne, C₂H₂, each carbon atom forms a triple bond with the other carbon atom and a single bond with one hydrogen atom).
4.3 Examples of Carbon Compounds and Their Bonds
| Compound | Formula | Bond Type(s) | Description |
|---|---|---|---|
| Methane | CH₄ | Four single bonds | Simplest alkane; primary component of natural gas |
| Ethene (Ethylene) | C₂H₄ | One double bond, four single | Plant hormone; used in the production of polyethylene |
| Ethyne (Acetylene) | C₂H₂ | One triple bond, two single | Used in welding torches due to its high flammability |
| Carbon Dioxide | CO₂ | Two double bonds | Greenhouse gas; essential for photosynthesis |
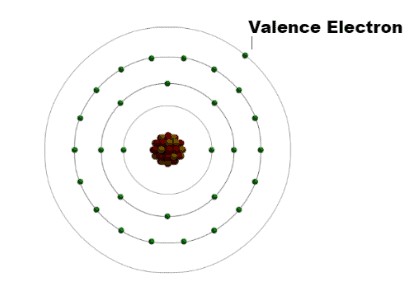
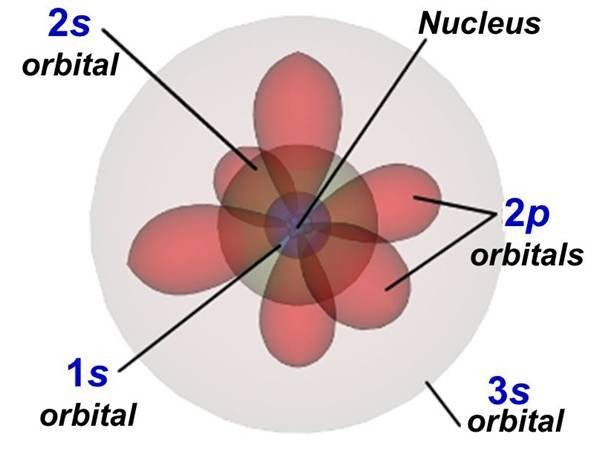
5. The Octet Rule and Carbon
The octet rule states that atoms tend to gain, lose, or share electrons in order to achieve a full valence shell with eight electrons. Carbon follows the octet rule by forming four covalent bonds, thereby surrounding itself with eight valence electrons.
5.1 Defining the Octet Rule
The octet rule is a guideline that helps predict how atoms will bond. Atoms are most stable when they have eight valence electrons, resembling the electron configuration of noble gases.
5.2 How Carbon Achieves an Octet
Carbon achieves an octet by forming four covalent bonds. Each bond contributes two electrons to carbon’s valence shell, resulting in a total of eight electrons. This stability makes carbon compounds abundant and versatile.
5.3 Exceptions to the Octet Rule
While carbon typically follows the octet rule, there are exceptions. In some compounds, carbon may have fewer than eight electrons around it, such as in carbocations (positively charged carbon ions) or carbenes (neutral molecules with a divalent carbon atom).
6. Lewis Structures and Carbon
Lewis structures, also known as electron dot diagrams, are visual representations of molecules showing the arrangement of atoms and the distribution of valence electrons. Drawing Lewis structures for carbon compounds helps illustrate how carbon satisfies the octet rule.
6.1 Introduction to Lewis Structures
Lewis structures use dots to represent valence electrons and lines to represent covalent bonds. These diagrams show how atoms are connected in a molecule and whether they have any lone pairs of electrons.
6.2 Drawing Lewis Structures for Carbon Compounds
- Determine the total number of valence electrons in the molecule by summing the valence electrons of each atom.
- Draw the skeletal structure with carbon as the central atom (if applicable) and connect the atoms with single bonds.
- Distribute the remaining electrons as lone pairs around the atoms, starting with the most electronegative atoms, to satisfy the octet rule.
- Form multiple bonds if necessary to ensure that each atom has an octet of electrons.
6.3 Examples of Lewis Structures for Carbon Compounds
- Methane (CH₄): Carbon has four valence electrons, and each hydrogen atom has one. The Lewis structure shows carbon forming four single bonds with four hydrogen atoms, satisfying the octet rule for carbon and the duet rule for hydrogen.
- Carbon Dioxide (CO₂): Carbon has four valence electrons, and each oxygen atom has six. The Lewis structure shows carbon forming two double bonds with two oxygen atoms, satisfying the octet rule for all atoms.
- Ethyne (C₂H₂): Each carbon atom has four valence electrons, and each hydrogen atom has one. The Lewis structure shows each carbon forming a triple bond with the other carbon and a single bond with one hydrogen, satisfying the octet rule for carbon and the duet rule for hydrogen.
7. Hybridization and Carbon Bonding
Hybridization is the mixing of atomic orbitals to form new hybrid orbitals suitable for bonding. Carbon undergoes hybridization to form sp³, sp², and sp hybrid orbitals, which determine the geometry and properties of its compounds.
7.1 Understanding Hybridization
Hybridization involves the combination of atomic orbitals (s, p, d) to form new hybrid orbitals with different shapes and energies. This process allows atoms to form stronger and more stable bonds.
7.2 sp³ Hybridization
In sp³ hybridization, one s orbital and three p orbitals mix to form four equivalent sp³ hybrid orbitals. This type of hybridization occurs when carbon forms four single bonds, such as in methane (CH₄). The geometry around the carbon atom is tetrahedral, with bond angles of approximately 109.5°.
7.3 sp² Hybridization
In sp² hybridization, one s orbital and two p orbitals mix to form three equivalent sp² hybrid orbitals, leaving one unhybridized p orbital. This type of hybridization occurs when carbon forms one double bond and two single bonds, such as in ethene (C₂H₄). The geometry around the carbon atom is trigonal planar, with bond angles of approximately 120°.
7.4 sp Hybridization
In sp hybridization, one s orbital and one p orbital mix to form two equivalent sp hybrid orbitals, leaving two unhybridized p orbitals. This type of hybridization occurs when carbon forms one triple bond and one single bond, such as in ethyne (C₂H₂). The geometry around the carbon atom is linear, with a bond angle of 180°.
8. Allotropes of Carbon
Carbon exists in various allotropic forms, each with unique physical and chemical properties due to different bonding arrangements. The most well-known allotropes of carbon include diamond, graphite, fullerenes, and graphene.
8.1 Defining Allotropes
Allotropes are different structural forms of the same element in the same physical state. The properties of allotropes can vary significantly due to differences in their atomic arrangements and bonding.
8.2 Diamond
In diamond, each carbon atom is sp³ hybridized and forms four single bonds with four other carbon atoms in a tetrahedral arrangement. This three-dimensional network structure makes diamond extremely hard and an excellent electrical insulator.
8.3 Graphite
In graphite, each carbon atom is sp² hybridized and forms three sigma bonds with three other carbon atoms in a trigonal planar arrangement. The unhybridized p orbitals form pi bonds, creating delocalized electrons that allow graphite to conduct electricity. Graphite consists of layers of carbon atoms arranged in hexagonal rings, which can slide over each other, making it a good lubricant.
8.4 Fullerenes
Fullerenes are spherical or ellipsoidal molecules composed of carbon atoms arranged in pentagons and hexagons. The most famous fullerene is buckminsterfullerene (C₆₀), which has a structure resembling a soccer ball. Fullerenes have unique electrical and mechanical properties and are used in various applications, including drug delivery and nanotechnology.
8.5 Graphene
Graphene is a single layer of carbon atoms arranged in a hexagonal lattice. Each carbon atom is sp² hybridized and forms three sigma bonds with three other carbon atoms. Graphene is incredibly strong, lightweight, and an excellent conductor of electricity and heat. It has numerous potential applications in electronics, energy storage, and composite materials.
9. Carbon in Organic Chemistry
Carbon is the backbone of organic chemistry, the study of carbon-containing compounds. The ability of carbon to form stable bonds with itself and other elements leads to an enormous variety of organic molecules with diverse structures and functions.
9.1 The Importance of Carbon in Organic Compounds
Carbon’s unique bonding properties make it essential for the formation of complex molecules necessary for life. Organic compounds include carbohydrates, lipids, proteins, and nucleic acids, which are the building blocks of living organisms.
9.2 Functional Groups
Functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. Common functional groups in organic chemistry include hydroxyl (-OH), carbonyl (C=O), carboxyl (-COOH), amino (-NH₂), and ether (-O-).
9.3 Isomerism
Isomers are molecules that have the same molecular formula but different structural arrangements. Isomerism contributes to the diversity of organic compounds, as different isomers can have different physical and chemical properties.
10. Real-World Applications of Carbon Compounds
Carbon compounds are used in a wide range of applications, from fuels and plastics to pharmaceuticals and electronics. Understanding the properties of carbon and its compounds is essential for developing new technologies and solving global challenges.
10.1 Fuels
Fossil fuels, such as coal, oil, and natural gas, are primarily composed of carbon and hydrogen. These fuels are used to generate energy for transportation, electricity, and heating.
10.2 Plastics
Plastics are polymers made from carbon-containing monomers. They are versatile materials used in packaging, construction, automotive, and consumer products.
10.3 Pharmaceuticals
Many pharmaceuticals are organic compounds containing carbon. These drugs are designed to interact with specific biological targets in the body to treat diseases and improve health.
10.4 Electronics
Carbon-based materials, such as graphene and carbon nanotubes, are used in electronic devices due to their excellent electrical conductivity and mechanical strength. They are used in transistors, sensors, and energy storage devices.
11. Consulting with Experts at HOW.EDU.VN
Navigating the complexities of chemistry, especially understanding valence electrons and carbon bonding, can be challenging. At HOW.EDU.VN, we offer direct access to leading Ph.D. experts ready to provide personalized guidance and answers to your most pressing questions.
11.1 Accessing Expert Knowledge
HOW.EDU.VN connects you with over 100 renowned Ph.D. experts across various fields. Our experts offer in-depth consultations tailored to your specific needs, whether you’re a student, professional, or simply curious.
11.2 Personalized Consultation Services
We understand that every individual has unique questions and challenges. Our consultation services are designed to provide personalized advice, ensuring you receive the most relevant and effective solutions.
11.3 Benefits of Consulting with Our Ph.D. Experts
- Save Time and Money: Avoid endless online searches and get straight to the answers you need.
- Gain Deep Insights: Benefit from the knowledge and experience of top experts in their fields.
- Ensure Accuracy: Rely on verified information and evidence-based advice.
- Get Practical Solutions: Receive actionable recommendations that you can implement immediately.
12. FAQs About Valence Electrons and Carbon
12.1 How Do You Calculate Valence Electrons?
Valence electrons can be determined using two primary methods: the periodic table and electron configuration.
- Periodic Table Method: Locate the element on the periodic table. The group number (vertical column) indicates the number of valence electrons for main group elements.
- Group 1: 1 valence electron
- Group 2: 2 valence electrons
- Group 13: 3 valence electrons
- Group 14: 4 valence electrons
- Group 15: 5 valence electrons
- Group 16: 6 valence electrons
- Group 17: 7 valence electrons
- Group 18: 8 valence electrons (except helium, which has 2)
- Electron Configuration Method: Write the electron configuration of the element. The number of electrons in the outermost shell (highest energy level) represents the number of valence electrons.
12.2 What is the 2 8 8 18 Rule in Chemistry?
The 2, 8, 8, 18 rule describes the maximum number of electrons that can occupy the electron shells of an atom. The first shell can hold up to 2 electrons, the second shell up to 8 electrons, the third shell up to 8 or 18 electrons, and the fourth shell up to 18 or 32 electrons. This rule helps predict the electron configuration and stability of atoms. The number of electrons in the nth shell is given by the formula 2n².
12.3 Where Can You Find the Number of Valence Electrons on the Periodic Table?
The number of valence electrons can be found by looking at the group number (vertical column) of the element in the periodic table. Main group elements in the same group have the same number of valence electrons.
12.4 How Do You Determine the Number of Valence Electrons for Beryllium?
Beryllium (Be) is in Group 2 of the periodic table. Therefore, beryllium has 2 valence electrons. Its electron configuration is 1s²2s², which also shows that it has 2 electrons in its outermost shell.
12.5 Why is Carbon So Important in Organic Chemistry?
Carbon’s significance in organic chemistry stems from its unique ability to form stable covalent bonds with itself and a variety of other elements, particularly hydrogen, oxygen, and nitrogen. This bonding versatility enables carbon to create a vast diversity of complex structures, including chains, rings, and three-dimensional arrangements. These structures serve as the foundation for a wide array of organic compounds, such as carbohydrates, lipids, proteins, and nucleic acids, which are essential for life.
12.6 How Does Carbon Achieve a Stable Octet?
Carbon achieves a stable octet, meaning eight electrons in its valence shell, by forming four covalent bonds with other atoms. Each covalent bond involves the sharing of two electrons, effectively contributing two electrons to carbon’s valence shell.
12.7 What is Hybridization and Why is it Important for Carbon?
Hybridization is the mixing of atomic orbitals to form new hybrid orbitals suitable for bonding. Carbon undergoes hybridization to form sp³, sp², and sp hybrid orbitals, which determine the geometry and properties of its compounds.
12.8 Can Carbon Form Ionic Bonds?
While carbon primarily forms covalent bonds, it can participate in ionic bonding under specific circumstances. For instance, in compounds like carbides, carbon can form ionic bonds with highly electropositive metals, such as aluminum or calcium.
12.9 What are the Common Allotropes of Carbon and How Do They Differ?
The most well-known allotropes of carbon include diamond, graphite, fullerenes, and graphene. Each allotrope exhibits distinct physical and chemical properties due to differences in their atomic arrangements and bonding.
12.10 How Do Functional Groups Influence the Properties of Organic Compounds?
Functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules.
Conclusion
Understanding how many valence electrons carbon has is fundamental to understanding its role in chemistry. With four valence electrons, carbon forms stable covalent bonds, leading to a vast array of organic compounds with diverse properties and applications. At HOW.EDU.VN, we are committed to providing expert guidance to help you master complex scientific concepts.
Are you facing challenges in understanding chemical bonding or other complex topics? Don’t struggle alone. Contact HOW.EDU.VN today and connect with our team of Ph.D. experts for personalized consultation services.
Contact Us:
- Address: 456 Expertise Plaza, Consult City, CA 90210, United States
- WhatsApp: +1 (310) 555-1212
- Website: HOW.EDU.VN
Let how.edu.vn be your trusted partner in achieving academic and professional success. Reach out now to get the expert advice you deserve.
