Navigating the world of chemistry often requires understanding fundamental concepts like How To Find Formal Charge, a concept crucial for predicting molecular structure and reactivity. At HOW.EDU.VN, we provide expert guidance on complex topics, helping you grasp the intricacies of chemical bonding and molecular behavior with ease. Dive into this detailed exploration to master formal charge calculation and its significance in chemical analysis, ensuring you have the knowledge to excel. Understanding the formula for formal charge is essential for predicting the stability and reactivity of molecules, making it a cornerstone of chemical education and research.
1. Understanding Formal Charge
Formal charge is a systematic method used in chemistry to allocate a charge to individual atoms within a molecule, assuming that electrons in all chemical bonds are shared equally between atoms, regardless of relative electronegativity. It serves as a critical tool for chemists, aiding in the prediction of molecular geometry and stability. While it is a simplified model and does not represent the actual charge distribution within a molecule (which can be influenced by electronegativity differences), formal charge helps in determining the most likely Lewis structure for a molecule or ion. This is because structures with minimal formal charges on atoms are generally more stable.
The concept of formal charge is intrinsically linked to understanding valence electrons, non-bonding electrons, and bonding electrons. It is essential for predicting the distribution of electrons in molecules and understanding their reactivity. While formal charge simplifies electron distribution and does not account for electronegativity differences, it provides valuable insights into molecular structure and reactivity. Understanding how to determine formal charge is crucial for anyone studying chemistry, whether you are a student, researcher, or professional in the field. The formal charge calculation helps to identify the most stable Lewis structures, assess molecular stability, and predict the reactivity of molecules.
To illustrate, consider ozone (O3). One possible Lewis structure shows one oxygen atom double-bonded to a central oxygen and another oxygen single-bonded to the central oxygen. Calculating formal charges can show that the central oxygen has a +1 formal charge, the double-bonded oxygen has no formal charge, and the single-bonded oxygen has a -1 formal charge.
This calculation guides chemists in predicting molecular structure, reactivity, and stability, and it serves as a foundational concept in chemistry. If you’re facing challenges understanding these concepts, remember that expert guidance is available. Connect with our seasoned Ph.Ds. at HOW.EDU.VN for personalized support and clarity. We also offer a collaborative environment where you can discuss complex problems with other learners and experts in the field.
2. Key Components of Formal Charge Calculation
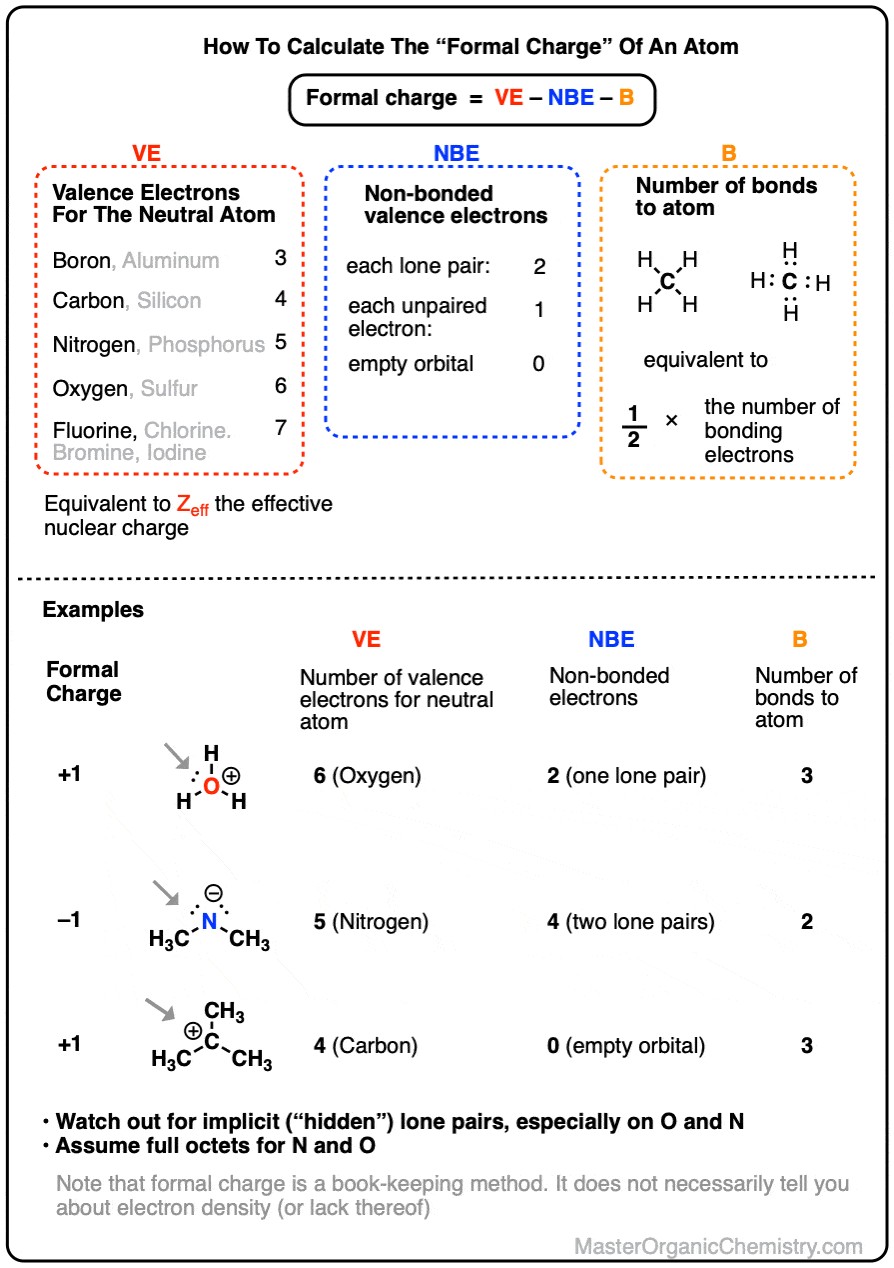
The formal charge calculation relies on three primary variables: valence electrons (VE), non-bonding electrons (NBE), and bonding electrons (B). Understanding each of these components is crucial for accurately determining formal charge and interpreting its significance in chemical structures.
2.1. Valence Electrons (VE)
Valence electrons are the electrons in the outermost shell of an atom and are crucial for chemical bonding. The number of valence electrons an atom possesses dictates its bonding behavior and chemical properties. For example, carbon (C) has four valence electrons, enabling it to form up to four covalent bonds. This tetravalency is fundamental to organic chemistry, allowing carbon to create a vast array of complex molecules. Oxygen (O), with six valence electrons, can form two covalent bonds and often has two lone pairs of electrons. Nitrogen (N), possessing five valence electrons, can form three covalent bonds and typically has one lone pair.
To identify the number of valence electrons, refer to the periodic table. Elements in the same group (vertical column) have the same number of valence electrons. For example, all elements in Group 1 (alkali metals) have one valence electron, while elements in Group 17 (halogens) have seven valence electrons.
Understanding the number of valence electrons is essential for drawing Lewis structures, predicting bonding patterns, and calculating formal charges. Accurately identifying valence electrons ensures correct Lewis structures and accurate formal charge calculations, which are vital for predicting molecular behavior and reactivity.
2.2. Non-Bonding Electrons (NBE)
Non-bonding electrons, also known as lone pairs, are valence electrons that are not involved in chemical bonds. These electrons reside on individual atoms and contribute to the overall electron density and charge distribution in a molecule. The presence of lone pairs can significantly influence the shape, polarity, and reactivity of a molecule.
For instance, in the water molecule (H2O), the oxygen atom has two lone pairs of electrons. These lone pairs repel the bonding pairs (electrons in the O-H bonds), causing the water molecule to adopt a bent shape rather than a linear one. This bent geometry gives water its polarity, making it an excellent solvent for ionic and polar compounds.
Identifying non-bonding electrons involves examining the Lewis structure of a molecule and counting the electrons that are not part of any bond. Each lone pair consists of two electrons. Accurately counting non-bonding electrons is essential for calculating formal charges and understanding the electronic properties of molecules. Non-bonding electrons contribute to the electron density around an atom, affecting its reactivity and interactions with other molecules. This understanding is critical in predicting chemical reactions and designing new molecules with specific properties.
2.3. Bonding Electrons (B)
Bonding electrons are the valence electrons involved in chemical bonds between atoms. These electrons are shared between atoms to form covalent bonds, holding the molecule together. The number of bonding electrons an atom shares influences its formal charge and the overall stability of the molecule.
In methane (CH4), each carbon atom shares four pairs of electrons with four hydrogen atoms, forming four covalent bonds. Each bond consists of two electrons, so there are eight bonding electrons around the carbon atom. In formal charge calculations, each atom is considered to “own” half of the bonding electrons in a bond.
To determine the number of bonding electrons for an atom, count the number of bonds it forms with other atoms and multiply by two (since each bond consists of two electrons). Alternatively, count the number of bonds directly, as each bond represents a shared pair of electrons. Accurately counting bonding electrons is essential for determining formal charges and assessing the electronic structure of molecules. Understanding bonding electrons provides insights into molecular stability, reactivity, and the types of chemical reactions a molecule is likely to undergo.
3. The Formal Charge Formula Explained
The formal charge formula is a straightforward yet powerful tool for determining the charge distribution within a molecule or ion. The formula is:
FC = VE – NBE – B
Where:
- FC is the formal charge of the atom.
- VE is the number of valence electrons of the neutral atom.
- NBE is the number of non-bonding electrons around the atom.
- B is the number of bonds around the atom.
To calculate the formal charge of an atom, start by identifying the number of valence electrons for the neutral atom using the periodic table. For example, nitrogen (N) is in Group 15 (or 5A), so it has five valence electrons. Next, count the number of non-bonding electrons (lone pairs) around the atom in the Lewis structure. Each lone pair consists of two electrons. Finally, count the number of bonds the atom forms with other atoms.
Subtract the number of non-bonding electrons and the number of bonds from the number of valence electrons to obtain the formal charge. The formal charge can be positive, negative, or zero. Atoms with formal charges closest to zero contribute most to the stability of the molecule. Understanding and applying the formal charge formula is crucial for predicting molecular structure, stability, and reactivity.
By identifying atoms with formal charges and understanding the distribution of charge within a molecule, chemists can make predictions about its behavior and interactions with other molecules. For complex problems, connect with our seasoned Ph.Ds at HOW.EDU.VN for personalized support and clarity. We also offer a collaborative environment where you can discuss complex problems with other learners and experts in the field.
4. Step-by-Step Guide to Calculating Formal Charge
Calculating formal charge involves a systematic approach that begins with constructing the correct Lewis structure for the molecule or ion. The Lewis structure shows all atoms, bonds, and lone pairs in the molecule, providing the necessary framework for formal charge calculations. Then, follow the steps outlined below:
4.1. Draw the Lewis Structure
The first step is to draw the Lewis structure of the molecule or ion. This involves arranging the atoms, placing the valence electrons around them, and forming bonds to satisfy the octet rule (or duet rule for hydrogen). For example, consider the Lewis structure of carbon dioxide (CO2). The carbon atom is in the center, double-bonded to each oxygen atom. Each oxygen atom also has two lone pairs of electrons.
Ensure that the Lewis structure follows the octet rule for most atoms (except for hydrogen, which follows the duet rule). The octet rule states that atoms tend to gain, lose, or share electrons to achieve a full outer shell of eight electrons. Once the Lewis structure is complete, proceed to the next step.
4.2. Identify Valence Electrons (VE)
Refer to the periodic table to determine the number of valence electrons for each atom in the molecule or ion. Valence electrons are the electrons in the outermost shell of an atom and dictate its bonding behavior. For example, carbon (C) has four valence electrons, oxygen (O) has six, and nitrogen (N) has five. List the number of valence electrons for each atom in the molecule. This information will be used in the formal charge formula.
4.3. Count Non-Bonding Electrons (NBE)
Count the number of non-bonding electrons (lone pairs) around each atom in the Lewis structure. Non-bonding electrons are valence electrons that are not involved in chemical bonds and reside on individual atoms as lone pairs. Each lone pair consists of two electrons. For example, in carbon dioxide (CO2), each oxygen atom has two lone pairs, so each oxygen has four non-bonding electrons. List the number of non-bonding electrons for each atom.
4.4. Count Bonds (B)
Count the number of bonds each atom forms with other atoms in the Lewis structure. A bond represents a shared pair of electrons between two atoms. For example, in carbon dioxide (CO2), the carbon atom forms four bonds (two double bonds) and each oxygen atom forms two bonds (one double bond). List the number of bonds for each atom.
4.5. Apply the Formal Charge Formula
Apply the formal charge formula FC = VE – NBE – B to each atom in the molecule or ion. Substitute the values for valence electrons (VE), non-bonding electrons (NBE), and bonds (B) into the formula and calculate the formal charge for each atom.
For example, in carbon dioxide (CO2):
- For carbon (C): FC = 4 (VE) – 0 (NBE) – 4 (B) = 0
- For oxygen (O): FC = 6 (VE) – 4 (NBE) – 2 (B) = 0
The formal charge of carbon and oxygen in carbon dioxide is zero, indicating a stable structure.
4.6. Check and Verify
After calculating the formal charges for all atoms, check and verify that the sum of the formal charges equals the overall charge of the molecule or ion. For neutral molecules, the sum of the formal charges should be zero. For ions, the sum of the formal charges should equal the charge of the ion. If the sum of the formal charges does not match the overall charge, re-examine the Lewis structure and calculations for any errors.
5. Formal Charge: Advanced Scenarios
While the basic formula for formal charge remains consistent, there are advanced scenarios where applying the formula requires careful consideration and a deeper understanding of molecular structure and bonding. These scenarios often involve resonance structures, expanded octets, and complex molecules with multiple atoms.
5.1. Resonance Structures
Resonance structures occur when a molecule or ion can be represented by two or more Lewis structures that differ only in the arrangement of electrons, not the arrangement of atoms. In such cases, the actual electronic structure of the molecule is a hybrid or average of all resonance structures. Formal charge plays a crucial role in evaluating the relative contribution of each resonance structure to the overall structure.
For example, consider the ozone molecule (O3), which has two resonance structures. In one structure, one oxygen atom forms a double bond with the central oxygen, while the other oxygen forms a single bond. In the second structure, the positions of the single and double bonds are reversed.
Calculating formal charges for each atom in both resonance structures reveals that the central oxygen has a +1 formal charge, one terminal oxygen has a -1 formal charge, and the other terminal oxygen has a 0 formal charge. The actual structure of ozone is a hybrid of these resonance structures, with the formal charges distributed among the oxygen atoms. The resonance structure with the lowest formal charges and the most electronegative atom bearing the negative charge is the major contributor.
5.2. Expanded Octets
Some atoms, particularly those in the third period and beyond, can accommodate more than eight electrons in their valence shell, forming expanded octets. This phenomenon is commonly observed in molecules such as sulfur hexafluoride (SF6) and phosphorus pentachloride (PCl5). Calculating formal charges in molecules with expanded octets requires careful consideration of the number of valence electrons and bonds around the central atom.
For example, in sulfur hexafluoride (SF6), the sulfur atom is bonded to six fluorine atoms. Sulfur has six valence electrons, and it forms six bonds. The formal charge on sulfur is calculated as:
FC = VE – NBE – B = 6 – 0 – 6 = 0
The formal charge on each fluorine atom is also zero, indicating a stable structure with minimal charge separation. Expanded octets allow certain molecules to exhibit unique bonding arrangements and properties, making them valuable in various chemical applications.
5.3. Complex Molecules
Calculating formal charges in complex molecules with multiple atoms and diverse bonding arrangements can be challenging. In such cases, it is essential to systematically analyze each atom, count valence electrons, non-bonding electrons, and bonds, and apply the formal charge formula.
For example, consider a complex organic molecule with multiple functional groups and heteroatoms. Start by drawing the Lewis structure, ensuring all atoms follow the octet rule (or expanded octet rule, if applicable). Then, identify each atom, count its valence electrons, non-bonding electrons, and bonds, and calculate the formal charge.
By systematically analyzing each atom and applying the formal charge formula, chemists can gain valuable insights into the charge distribution, stability, and reactivity of complex molecules.
6. Common Pitfalls and How to Avoid Them
Calculating formal charge is a fundamental skill in chemistry, but it is easy to make mistakes if you are not careful. Understanding common pitfalls and how to avoid them can improve accuracy and deepen understanding.
6.1. Incorrect Lewis Structures
A common pitfall is drawing incorrect Lewis structures. An accurate Lewis structure is essential for calculating formal charges correctly. Ensure that all atoms follow the octet rule (or duet rule for hydrogen) and that the total number of valence electrons matches the sum of valence electrons for all atoms in the molecule or ion.
6.2. Miscounting Valence Electrons
Another common mistake is miscounting valence electrons. Refer to the periodic table to accurately determine the number of valence electrons for each atom. Remember that elements in the same group have the same number of valence electrons. Understanding the periodic table trends can prevent this error.
6.3. Overlooking Lone Pairs
It is easy to overlook lone pairs of electrons, especially in complex molecules. Ensure that all non-bonding electrons are accounted for in the Lewis structure. Remember that each lone pair consists of two electrons. Double-checking the Lewis structure can prevent overlooking lone pairs.
6.4. Incorrectly Counting Bonds
Incorrectly counting bonds can lead to errors in formal charge calculations. Count the number of bonds each atom forms with other atoms in the Lewis structure. A single bond consists of one shared pair of electrons, a double bond consists of two shared pairs of electrons, and a triple bond consists of three shared pairs of electrons.
6.5. Forgetting the Overall Charge
Always verify that the sum of the formal charges equals the overall charge of the molecule or ion. For neutral molecules, the sum of the formal charges should be zero. For ions, the sum of the formal charges should equal the charge of the ion. If the sum of the formal charges does not match the overall charge, re-examine the Lewis structure and calculations for any errors.
7. The Significance of Formal Charge in Chemistry
Formal charge is a crucial concept in chemistry that provides valuable insights into molecular structure, stability, and reactivity. Understanding the significance of formal charge allows chemists to make informed predictions about the behavior of molecules and their interactions with other substances.
7.1. Predicting Molecular Stability
Formal charge is used to predict the relative stability of different Lewis structures for a molecule or ion. The Lewis structure with the lowest formal charges on the atoms is generally the most stable. Structures with minimal charge separation are energetically more favorable than those with large formal charges.
7.2. Understanding Molecular Reactivity
Formal charge can provide insights into the reactivity of molecules. Atoms with large formal charges are more likely to participate in chemical reactions to reduce or neutralize the charge. For example, a molecule with a positively charged atom may be susceptible to nucleophilic attack, while a molecule with a negatively charged atom may be prone to electrophilic attack.
7.3. Evaluating Resonance Structures
In molecules with resonance structures, formal charge helps evaluate the relative contribution of each resonance structure to the overall structure. The resonance structure with the lowest formal charges and the most electronegative atom bearing the negative charge is the major contributor. Understanding the relative contributions of resonance structures is essential for predicting the properties and behavior of molecules.
7.4. Designing New Molecules
Formal charge plays a role in the design of new molecules with specific properties. By understanding how formal charges affect molecular stability and reactivity, chemists can design molecules with desired characteristics. For instance, formal charge considerations can guide the design of catalysts, drugs, and materials with tailored properties.
8. Case Studies: Real-World Applications of Formal Charge
To illustrate the practical applications of formal charge, consider the following real-world case studies:
8.1. Ozone Depletion
Ozone (O3) in the Earth’s stratosphere absorbs harmful ultraviolet (UV) radiation from the sun, protecting life on Earth. However, ozone can be depleted by various chemical reactions, particularly those involving chlorine atoms. Formal charge calculations help understand the electronic structure of ozone and its interactions with chlorine atoms.
8.2. Drug Design
Formal charge is used in drug design to optimize the properties of drug candidates. By analyzing the formal charges on different atoms in a drug molecule, chemists can predict how the drug will interact with its biological target. Formal charge considerations can guide the design of drugs with improved binding affinity, selectivity, and pharmacokinetic properties.
8.3. Material Science
Formal charge plays a role in material science to understand the properties of materials such as polymers, ceramics, and semiconductors. By analyzing the formal charges on atoms in a material, scientists can predict its electronic, optical, and mechanical properties. Formal charge considerations can guide the design of materials with tailored properties for various applications, such as electronics, energy storage, and aerospace.
These real-world case studies demonstrate the versatility and importance of formal charge in various scientific disciplines.
9. Elevate Your Expertise with HOW.EDU.VN
At HOW.EDU.VN, we connect you with over 100 globally recognized Ph.Ds and experts, offering unparalleled access to top-tier knowledge and support. Whether you are grappling with complex formal charge calculations or need assistance with any scientific challenge, our experts are ready to provide clear, personalized guidance. Unlock your potential with insights from the world’s leading minds, all conveniently accessible through our platform. Contact us today and transform your understanding of chemistry.
9.1. Access to Leading Experts
HOW.EDU.VN provides access to a diverse network of leading experts in various fields, including chemistry, physics, biology, and more. Our experts are Ph.Ds and seasoned professionals with extensive experience in their respective domains. By connecting with our experts, you can gain access to specialized knowledge, insights, and guidance that can help you excel in your academic or professional endeavors.
9.2. Personalized Support
We understand that every individual has unique needs and learning preferences. That’s why we offer personalized support tailored to your specific requirements. Whether you need help with a specific concept, guidance on a research project, or advice on career development, our experts are here to provide customized assistance.
9.3. Collaborative Environment
HOW.EDU.VN fosters a collaborative environment where you can interact with other learners and experts in your field. Our platform provides opportunities for discussions, Q&A sessions, and collaborative projects. By engaging with others, you can broaden your perspectives, enhance your learning, and build valuable connections.
10. Frequently Asked Questions (FAQ) about Formal Charge
Q1: What is formal charge, and why is it important?
Formal charge is a concept used to assign charges to individual atoms within a molecule, assuming equal sharing of electrons in chemical bonds. It helps predict molecular geometry, stability, and reactivity, making it a fundamental tool in chemistry.
Q2: How do I calculate the formal charge of an atom?
The formal charge is calculated using the formula: FC = VE – NBE – B, where VE is the number of valence electrons, NBE is the number of non-bonding electrons, and B is the number of bonds around the atom.
Q3: What are valence electrons, and how do I determine them?
Valence electrons are the electrons in the outermost shell of an atom. You can determine them by referring to the periodic table; elements in the same group have the same number of valence electrons.
Q4: What are non-bonding electrons (NBE), and how do I count them?
Non-bonding electrons, or lone pairs, are valence electrons not involved in chemical bonds. Count the electrons not part of any bond in the Lewis structure; each lone pair consists of two electrons.
Q5: What is the significance of formal charge in predicting molecular stability?
The Lewis structure with the lowest formal charges on the atoms is generally the most stable. Structures with minimal charge separation are energetically more favorable.
Q6: How does formal charge relate to molecular reactivity?
Atoms with large formal charges are more likely to participate in chemical reactions to reduce or neutralize the charge. Positive charges may attract nucleophiles, while negative charges may attract electrophiles.
Q7: Can formal charge help in evaluating resonance structures?
Yes, in molecules with resonance structures, formal charge helps evaluate the contribution of each structure. The structure with the lowest formal charges and the most electronegative atom bearing the negative charge is the major contributor.
Q8: How is formal charge used in drug design?
In drug design, formal charges help optimize drug candidate properties by predicting how the drug will interact with its biological target, improving binding affinity, selectivity, and pharmacokinetic properties.
Q9: How does formal charge relate to expanded octets?
Atoms in the third period and beyond can accommodate more than eight electrons in their valence shell. Calculating formal charges in molecules with expanded octets requires careful consideration of the number of valence electrons and bonds around the central atom.
Q10: Where can I find expert help if I’m struggling with formal charge calculations?
HOW.EDU.VN connects you with over 100 globally recognized Ph.Ds and experts who can provide personalized guidance and support. Contact us today to transform your understanding of chemistry.
Mastering how to determine formal charge is essential for anyone studying or working in chemistry, enabling accurate predictions about molecular behavior and reactivity.
Don’t navigate complex scientific challenges alone. At HOW.EDU.VN, we connect you with over 100 globally recognized Ph.Ds and experts ready to provide personalized guidance. Whether you’re grappling with intricate formal charge calculations or seeking insights into advanced chemical concepts, our platform offers unparalleled access to top-tier knowledge and support. Let our experts transform your understanding and unlock your potential.
Ready to take your understanding of chemistry to the next level? Contact HOW.EDU.VN today and connect with a Ph.D. expert who can provide the personalized guidance you need to excel.
Address: 456 Expertise Plaza, Consult City, CA 90210, United States
WhatsApp: +1 (310) 555-1212
Website: how.edu.vn
 Formal charge formula how to calculate the formal charge of an atom master organic chemistry
Formal charge formula how to calculate the formal charge of an atom master organic chemistry