The weight of 1 cup of water is approximately 8 ounces, equivalent to a half pound; however, this can vary due to factors such as temperature and measurement accuracy, but as HOW.EDU.VN, we’re here to clarify this with expert insights. Understanding this basic measurement is crucial for cooking, baking, and scientific applications. Discover more detailed explanations and practical tips below, and don’t hesitate to reach out to our team of experts for personalized assistance with related topics such as fluid density, culinary measurements, and nutritional analysis.
Here are 5 search intentions for the keyword “How Much Does 1 Cup Of Water Weigh”:
- Basic Information: Users want a quick and simple answer to the question.
- Accuracy and Precision: Users need precise measurements for cooking, baking, or scientific purposes.
- Factors Affecting Weight: Users are curious about variables that can change the weight of water.
- Comparison with Other Liquids: Users want to know how the weight of water compares to other common liquids.
- Practical Applications: Users seek guidance on how to accurately measure water in real-world scenarios.
1. Understanding Ounces and Fluid Ounces
You may have encountered the terms “ounce” and “fluid ounce,” which, while similar, refer to different measurements. An ounce (oz) is a unit of weight, while a fluid ounce (fl oz) is a unit of volume.
- Ounce (oz) = weight
- Fluid ounce (fl oz) = volume
For instance, consider water. Eight fluid ounces (volume) of water equals approximately 8 ounces (weight). Therefore, if measured accurately, 1 cup of water will weigh about 8 ounces on a scale.
2. The Weight of Water vs. Other Liquids
The weight of 1 cup of a liquid depends on its density. Water is a standard reference, but liquids with higher densities, like honey, weigh more. Conversely, liquids with lower densities, such as canola or vegetable oil, weigh less.
- Water: 1 cup (8 fl oz) weighs 8 oz
- Honey: 1 cup (8 fl oz) weighs 12 oz
- Canola Oil: 1 cup (8 fl oz) weighs about 7.1 oz
2.1. Density Explained
Density is a measure of mass per unit volume. Liquids with higher densities have more mass packed into the same volume, making them heavier. This is why honey weighs more than water, and oil weighs less.
2.2. Practical Examples of Density Differences
If you’ve ever mixed oil and water, you’ve likely noticed that oil floats on top. This happens because oil is less dense than water. The same principle applies to other liquids with varying densities.
3. Tools for Accurate Measurement
Accurate measurements require the right tools and proper techniques. Whether measuring volume or weight, precision is key in cooking, baking, and scientific applications.
3.1. Liquid Measuring Cups (Volume)
Liquid measuring cups are typically made of glass or clear plastic, allowing you to see the liquid level and ensure an accurate measurement.
3.1.1. Tips for Measuring Liquids
- Use the largest, appropriate standard measuring container.
- Place the measuring container on a flat surface.
- Pour the liquid into the container until it reaches the desired level.
- Read the measurement at eye level when using a clear container. For metal containers, look inside while filling to the desired level.
3.2. Dry Measuring Cups (Volume)
Dry measuring cups are usually made of plastic or metal and come in various sizes. These are ideal for measuring dry and solid ingredients when a scale is not available.
3.2.1. Tips for Measuring Dry Ingredients
- Use the largest, appropriate standard measuring container.
- Spoon the ingredient lightly into the measuring container.
- Exception: Pack brown sugar firmly into the measuring container to maintain its shape when emptied.
- Fill the measuring container overflowing, then level off with a straight-edged spatula or knife.
- Avoid shaking or tapping the measuring container.
3.3. Kitchen Scales (Weight)
Weighing is a quicker, easier, and more accurate method for measuring larger amounts of dry and solid ingredients. Kitchen scales come in traditional and electronic models.
3.3.1. How to Use a Kitchen Scale
- Select the unit of measurement (ounces or grams) listed in your recipe.
- If using a bowl or container, place it on the scale and press the “zero” or “tare” button to exclude its weight from the final measurement.
- Alternatively, place an empty bowl or container on the scale before turning it on; it will automatically tare to zero.
4. Practical Tips for the Kitchen
Navigating weight and volume in the kitchen can be simplified with a few key considerations.
4.1. Ingredient Amounts in Recipes
Recipes typically list ingredient amounts by weight and volume. Pay close attention to the listed units and use the appropriate tools for accurate measurements.
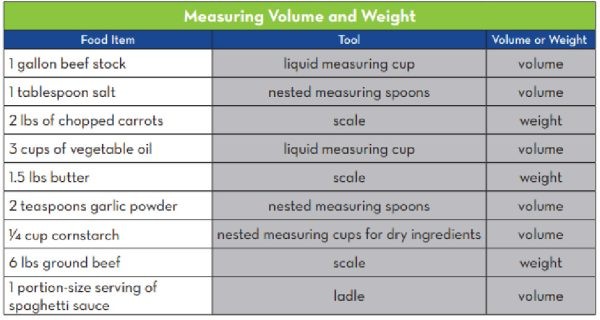 Measuring Volume and Weight
Measuring Volume and Weight
4.2. Weight-to-Volume Equivalents
Remember that weight-to-volume equivalents vary for each ingredient. For example, 1 cup of raw broccoli florets weighs approximately 3.25 ounces, while 1 cup of raw spinach weighs about 1 ounce. Consult resources like the Food Buying Guide for Child Nutrition Programs – Yield Tables for specific conversions.
4.3. Minimizing Measurements
Use tools that minimize the number of measurements needed. For instance, if a recipe calls for 7 teaspoons of salt, measure 2 tablespoons and 1 teaspoon instead. This is also equivalent to 1/8 cup plus 1 teaspoon.
4.4. Quick Reference Guides
The Basics at a Glance poster offers quick information on recipe abbreviations, measurement conversions, portioning tools, and fraction-to-decimal equivalents. Download and print this resource for easy reference in your kitchen.
5. CACFP Meal Service Guidelines
In the CACFP (Child and Adult Care Food Program) Meal Pattern, some meal components are listed by volume, while others are listed by weight. Adhere to the specified measurement requirements.
- Fluid milk is listed in fluid ounces (volume). A 6 fluid-ounce serving for 3–5-year-olds equals ¾ cup.
- Meats are listed by weight (ounces), while some meat alternates are listed by volume (e.g., ¼ cup cooked dried beans for a 1-2-year-old serving at lunch or supper).
- Grains are listed in ounce equivalents or by volume (e.g., ¼ cup of pasta for a 3-5-year-old).
- Vegetables and fruits are listed in cups (volume).
5.1. Ensuring Correct Minimum Requirements
To ensure children receive the correct minimum requirements, follow these guidelines:
- Use the correct measuring tools.
- Follow the recipe instructions, which specify the correct serving size (e.g., ½ cup of broccoli) and may recommend specific tools.
- Portion out the correct amounts by leveling the food. Filling an 8 oz ladle with soup ensures you are serving the right amount of 8 fluid ounces.
For additional tips, refer to ICN’s November Mealtime Memo.
6. The Science Behind Water Weight: A Deeper Dive
To fully grasp the concept of how much 1 cup of water weighs, we need to delve into the scientific principles that govern these measurements. Water, chemically represented as H2O, has a unique molecular structure that influences its density and, consequently, its weight per unit volume.
6.1. Molecular Structure and Density
Water molecules are polar, meaning they have a slightly positive charge on the hydrogen atoms and a slightly negative charge on the oxygen atom. This polarity allows water molecules to form hydrogen bonds with each other, creating a relatively dense structure.
6.1.1. Hydrogen Bonding
Hydrogen bonds are relatively weak compared to covalent bonds but are strong enough to hold water molecules close together. This close proximity contributes to water’s density.
6.1.2. Density Variations with Temperature
The density of water is not constant across all temperatures. Water is densest at approximately 4 degrees Celsius (39.2 degrees Fahrenheit). As water cools from this temperature, it becomes less dense, which is why ice floats on water.
6.2. Impurities and Their Impact
The purity of water also affects its weight. Dissolved minerals and other impurities can increase the density of water, leading to a slightly higher weight per cup.
6.2.1. Distilled vs. Tap Water
Distilled water, which is nearly pure H2O, will have a slightly lower weight per cup compared to tap water, which contains dissolved minerals and chemicals.
6.2.2. Salinity
In marine environments, the salinity (salt content) significantly increases the density of water. A cup of seawater will weigh more than a cup of freshwater.
6.3. Altitude and Atmospheric Pressure
Altitude and atmospheric pressure can also influence the weight of water, albeit minimally. Higher altitudes have lower atmospheric pressure, which can slightly reduce the density of water.
6.3.1. Boiling Point Variations
At higher altitudes, water boils at a lower temperature. This is because the lower atmospheric pressure requires less energy for water molecules to escape into the gaseous phase.
6.3.2. Practical Implications for Cooking
When cooking at high altitudes, you may need to adjust recipes and cooking times due to the lower boiling point of water.
7. Culinary Applications: Ensuring Precision in Recipes
In culinary arts, accuracy in measurements can be the difference between a delightful dish and a culinary disaster. Understanding the weight of water and other liquids is essential for consistent and reliable results.
7.1. Baking
Baking is a science as much as it is an art. Precise measurements are crucial for the chemical reactions that make baked goods rise and achieve the desired texture.
7.1.1. Hydration Levels
The hydration level of dough, for example, is critical in bread making. Too much or too little water can affect the gluten development and the final texture of the bread.
7.1.2. Liquid-to-Dry Ratios
Maintaining the correct liquid-to-dry ingredient ratios is also vital in cakes, pastries, and other baked goods.
7.2. Sauces and Soups
In sauce and soup preparation, the water content affects the consistency, flavor concentration, and overall balance of the dish.
7.2.1. Stock and Broth
The amount of water used in making stock and broth influences the intensity of the flavor. Using too much water can result in a weak, diluted flavor.
7.2.2. Reduction Techniques
Reduction, a technique used to concentrate flavors and thicken sauces, relies on evaporating water. Knowing the initial water content helps in predicting the final consistency and flavor profile.
7.3. Beverage Preparation
Whether brewing coffee, making tea, or mixing cocktails, the water-to-ingredient ratio is essential for achieving the desired taste and strength.
7.3.1. Coffee Brewing
The ratio of coffee grounds to water affects the extraction rate and the final flavor of the coffee. Too much water can lead to a weak, under-extracted brew, while too little water can result in a strong, bitter cup.
7.3.2. Tea Infusion
Similarly, the water temperature and steeping time in tea preparation influence the release of tannins and other compounds that contribute to the tea’s flavor and aroma.
8. Health and Hydration: Water Intake and Its Importance
Water is essential for life, and understanding how much water we consume is vital for maintaining good health. Knowing the weight of a cup of water can help you track your daily fluid intake more accurately.
8.1. Daily Water Requirements
The general recommendation is to drink about 8 cups (64 ounces) of water per day, but individual needs vary based on factors like activity level, climate, and overall health.
8.1.1. Factors Influencing Water Needs
Athletes and individuals who engage in strenuous physical activity need more water to replace fluids lost through sweat. Similarly, people living in hot climates need to drink more water to stay hydrated.
8.1.2. Monitoring Fluid Intake
Tracking your water intake can help ensure you’re meeting your daily hydration goals. Using a measuring cup to portion your water can provide a more accurate estimate of your consumption.
8.2. Benefits of Proper Hydration
Staying adequately hydrated offers numerous health benefits, including improved digestion, better cognitive function, and enhanced physical performance.
8.2.1. Digestion and Nutrient Absorption
Water aids in the digestion process by helping to break down food and transport nutrients throughout the body.
8.2.2. Cognitive Function
Dehydration can impair cognitive function, leading to fatigue, difficulty concentrating, and reduced mental performance.
8.2.3. Physical Performance
Proper hydration is essential for maintaining optimal physical performance. Dehydration can lead to muscle cramps, fatigue, and decreased endurance.
8.3. Recognizing Dehydration
Being able to recognize the signs of dehydration is crucial for taking prompt action to replenish fluids. Common symptoms include thirst, dry mouth, dark urine, and dizziness.
8.3.1. Severe Dehydration
In severe cases, dehydration can lead to more serious complications, such as heatstroke, seizures, and even death.
8.3.2. Hydration Strategies
Drinking water regularly throughout the day, especially during and after physical activity, is a simple yet effective strategy for preventing dehydration.
9. Scientific and Industrial Applications
Beyond the kitchen and personal health, the weight of water is a critical measurement in various scientific and industrial applications.
9.1. Chemistry
In chemistry, accurate measurements of water are essential for preparing solutions, conducting experiments, and analyzing results.
9.1.1. Molarity and Molality
Molarity (moles per liter) and molality (moles per kilogram) are common units of concentration used in chemistry. Accurate water measurements are crucial for calculating these values.
9.1.2. Titration
Titration, a technique used to determine the concentration of a substance, often involves measuring the volume of a liquid, such as water.
9.2. Environmental Science
Environmental scientists use water measurements to study ecosystems, monitor water quality, and assess the impact of pollution.
9.2.1. Water Sampling
Collecting and analyzing water samples require precise volume measurements to ensure accurate results.
9.2.2. Hydrology
Hydrology, the study of water movement and distribution, relies on accurate measurements of precipitation, evaporation, and runoff.
9.3. Manufacturing
Many manufacturing processes use water as a solvent, coolant, or cleaning agent. Accurate water measurements are essential for quality control and process optimization.
9.3.1. Pharmaceutical Industry
In the pharmaceutical industry, precise water measurements are critical for manufacturing drugs and other medical products.
9.3.2. Food and Beverage Industry
The food and beverage industry relies on accurate water measurements for producing consistent and safe products.
10. Expert Insights from HOW.EDU.VN: Common Measurement Mistakes and How to Avoid Them
Even with the right tools, measurement errors can occur. As HOW.EDU.VN, we’ve compiled expert insights to help you avoid common pitfalls and ensure accuracy in your measurements.
10.1. Common Mistakes in Measuring Volume
Measuring volume accurately requires attention to detail. Common mistakes include using the wrong type of measuring cup, not leveling dry ingredients, and failing to read liquid measurements at eye level.
10.1.1. Using the Wrong Measuring Cup
Using a liquid measuring cup for dry ingredients (or vice versa) can lead to significant errors. Liquid measuring cups are designed to measure volume, while dry measuring cups are designed to measure quantity.
10.1.2. Not Leveling Dry Ingredients
Failing to level dry ingredients can result in inconsistent measurements. Always use a straight-edged spatula or knife to level off dry ingredients after filling the measuring cup.
10.1.3. Not Reading at Eye Level
When measuring liquids, always read the measurement at eye level to avoid parallax errors. Parallax occurs when the angle of observation distorts the perceived measurement.
10.2. Common Mistakes in Measuring Weight
Weighing ingredients seems straightforward, but errors can still occur. Common mistakes include not taring the scale, using the wrong units, and not calibrating the scale regularly.
10.2.1. Not Taring the Scale
Failing to tare the scale before adding ingredients can result in inaccurate measurements. Always tare the scale to zero after placing a container on it.
10.2.2. Using the Wrong Units
Using the wrong units (e.g., grams instead of ounces) can lead to significant errors. Double-check the recipe and the scale settings to ensure you’re using the correct units.
10.2.3. Not Calibrating the Scale Regularly
Scales can drift over time, leading to inaccurate measurements. Calibrate your scale regularly using calibration weights to ensure accuracy.
10.3. Expert Tips for Accurate Measurements
To ensure accurate measurements every time, follow these expert tips:
10.3.1. Use High-Quality Measuring Tools
Invest in high-quality measuring cups, spoons, and scales for consistent and reliable results.
10.3.2. Double-Check Measurements
Before adding ingredients, double-check your measurements to catch any errors.
10.3.3. Keep Measuring Tools Clean
Clean measuring tools regularly to prevent buildup that can affect accuracy.
By understanding the science behind water weight, following best practices for measuring, and avoiding common mistakes, you can ensure accuracy in your cooking, baking, and scientific endeavors.
11. How Temperature Affects the Weight of Water: Detailed Analysis
The temperature of water has a notable impact on its density and, consequently, its weight. Understanding these nuances is particularly crucial for precise scientific and culinary applications.
11.1. Density and Temperature Relationship
Water’s density is at its highest at approximately 4°C (39.2°F). As water heats up or cools down from this point, its density decreases. This phenomenon is due to the behavior of water molecules and their interactions at different temperatures.
11.1.1. Molecular Behavior at Different Temperatures
At lower temperatures (below 4°C), water molecules form a more structured lattice due to hydrogen bonding, resulting in expansion and decreased density. As water warms up, the kinetic energy of the molecules increases, disrupting some of these hydrogen bonds and allowing the molecules to pack more closely together, increasing density until it reaches 4°C.
11.1.2. Practical Examples
- Cold Water: A cup of ice-cold water might weigh slightly less than a cup of water at room temperature.
- Hot Water: Similarly, a cup of boiling water will be less dense and weigh less than a cup of water at its densest state (4°C).
11.2. Specific Weight Variations with Temperature
While the changes in weight due to temperature are relatively small for typical household measurements, they become significant in scientific contexts where precision is paramount.
11.2.1. Quantitative Analysis
For instance, the density of water at 0°C (32°F) is approximately 0.99984 g/mL, while at 25°C (77°F) it is about 0.99705 g/mL. This difference, though small, can affect calculations and experimental outcomes.
11.2.2. Impact on Measurements
In laboratory settings, these variations are accounted for using temperature-compensated instruments and reference tables.
11.3. Implications for Cooking and Baking
In cooking and baking, temperature-related density changes can influence ingredient interactions and final product quality.
11.3.1. Yeast Activation
For example, the temperature of water used to activate yeast can affect its activity. Warmer water accelerates yeast activity, but excessively hot water can kill the yeast.
11.3.2. Dough Consistency
The temperature of water used in dough preparation also influences gluten development and overall dough consistency.
11.4. Addressing Temperature Variations in Measurements
To minimize the impact of temperature variations, consider these best practices:
11.4.1. Use Consistent Temperatures
When following recipes, try to use ingredients at consistent temperatures to minimize density variations.
11.4.2. Temperature-Compensated Instruments
In scientific settings, use temperature-compensated instruments to account for density changes.
11.4.3. Refer to Density Tables
Consult density tables for water at different temperatures to make precise adjustments when necessary.
By understanding and accounting for the effects of temperature on water density, you can achieve more accurate and reliable results in various applications.
12. The Role of Dissolved Substances: How They Affect Water Weight
The weight of water can also be influenced by the presence of dissolved substances. Understanding how these substances affect water’s density is crucial for accurate measurements and various applications.
12.1. Dissolved Minerals
Tap water, unlike distilled water, contains dissolved minerals such as calcium, magnesium, and sodium. These minerals increase the density of water, leading to a slightly higher weight per unit volume.
12.1.1. Mineral Content in Tap Water
The exact mineral content varies depending on the water source and treatment processes. However, the presence of these minerals consistently increases the density compared to pure water.
12.1.2. Practical Implications
For most household applications, the difference in weight between tap water and distilled water is negligible. However, in scientific experiments requiring high precision, it becomes relevant.
12.2. Salts and Salinity
The presence of salts, particularly sodium chloride (NaCl), significantly increases water’s density. This is most evident in seawater, which has a much higher density than freshwater.
12.2.1. Salinity Levels in Seawater
Seawater typically contains about 3.5% salt, which substantially increases its density. As a result, a cup of seawater weighs noticeably more than a cup of freshwater.
12.2.2. Environmental Applications
In marine biology and oceanography, salinity levels are critical for understanding ocean currents, marine life distribution, and other environmental factors.
12.3. Sugars and Other Dissolved Solids
Dissolving sugars and other solids in water also increases its density. This principle is used in various food and beverage applications.
12.3.1. Sugar Solutions
For example, a cup of sugar syrup weighs more than a cup of plain water due to the added sugar content.
12.3.2. Beverage Production
In beverage production, the sugar content is carefully controlled to achieve the desired density and sweetness levels.
12.4. Measuring and Accounting for Dissolved Substances
To accurately measure water with dissolved substances, consider these strategies:
12.4.1. Use Calibrated Instruments
Use calibrated instruments such as hydrometers or density meters to measure the density of the solution.
12.4.2. Reference Tables
Consult reference tables that provide density values for various solutions at different concentrations.
12.4.3. Control for Purity
In scientific experiments, use purified water (e.g., distilled or deionized water) to minimize the impact of dissolved substances.
By understanding how dissolved substances affect water’s weight, you can make more accurate measurements and achieve more reliable results in various applications.
13. Expert Insights on Measurement Best Practices for Water
Accurate measurement of water is essential in various fields, from cooking to chemistry. Here, we present expert insights and best practices to ensure precision and reliability in your measurements.
13.1. Choosing the Right Measuring Tool
The first step in accurate measurement is selecting the appropriate tool. Different tools are designed for specific purposes, and using the wrong one can lead to significant errors.
13.1.1. Liquid Measuring Cups vs. Dry Measuring Cups
- Liquid Measuring Cups: Ideal for measuring liquids accurately. These cups typically have a spout for easy pouring and are calibrated to measure volume.
- Dry Measuring Cups: Designed for measuring dry ingredients. These cups are filled to the top and leveled off for accurate measurement.
13.1.2. Kitchen Scales
Kitchen scales are essential for measuring weight accurately. Digital scales are preferred for their precision and ease of use.
13.2. Calibration and Maintenance of Measuring Tools
Regular calibration and maintenance are crucial for ensuring the accuracy of measuring tools.
13.2.1. Calibrating Kitchen Scales
Calibrate digital scales regularly using calibration weights. Follow the manufacturer’s instructions for calibration.
13.2.2. Cleaning Measuring Cups and Spoons
Clean measuring cups and spoons thoroughly after each use to prevent residue buildup that can affect accuracy.
13.3. Techniques for Accurate Measurement
Employing proper techniques is essential for obtaining accurate measurements.
13.3.1. Measuring Liquids
- Place the measuring cup on a flat surface.
- Pour the liquid slowly until it reaches the desired level.
- Read the measurement at eye level to avoid parallax errors.
13.3.2. Measuring Dry Ingredients
- Use dry measuring cups.
- Spoon the ingredient lightly into the measuring cup.
- Level off with a straight-edged spatula or knife.
- Avoid shaking or tapping the measuring cup.
13.4. Considering Environmental Factors
Environmental factors such as temperature and humidity can also affect measurement accuracy.
13.4.1. Temperature Control
Maintain consistent temperatures when measuring water and other liquids to minimize density variations.
13.4.2. Humidity Control
High humidity can affect the weight of dry ingredients. Store dry ingredients in airtight containers to prevent moisture absorption.
13.5. Utilizing Reference Materials
Reference materials such as density tables and conversion charts can be valuable resources for accurate measurement.
13.5.1. Density Tables for Water
Consult density tables for water at different temperatures to make precise adjustments when necessary.
13.5.2. Conversion Charts
Use conversion charts to convert between different units of measurement (e.g., ounces to milliliters).
By following these expert insights and best practices, you can ensure accurate and reliable measurements of water in various applications.
14. FAQ: Frequently Asked Questions About the Weight of Water
To further clarify the topic of how much 1 cup of water weighs, here are some frequently asked questions with detailed answers.
Q1: How much does 1 cup of water weigh in ounces?
A1: One cup of water typically weighs about 8 ounces. This is based on the standard U.S. measurement where 1 fluid ounce of water is approximately equal to 1 ounce in weight.
Q2: Does the temperature of water affect its weight?
A2: Yes, the temperature of water does affect its weight, albeit slightly. Water is densest at around 4°C (39.2°F). As it heats up or cools down from this point, its density decreases, leading to slight variations in weight.
Q3: Does tap water weigh the same as distilled water?
A3: No, tap water typically weighs slightly more than distilled water. Tap water contains dissolved minerals and chemicals, which increase its density compared to pure distilled water.
Q4: How does the weight of water compare to other liquids like oil or honey?
A4: The weight of water differs from other liquids due to differences in density. Liquids with higher densities, like honey, weigh more per cup, while liquids with lower densities, like oil, weigh less.
Q5: Why is it important to accurately measure water in cooking and baking?
A5: Accurate measurement of water is crucial in cooking and baking because it affects the consistency, texture, and overall outcome of the recipe. Precise water measurements ensure that the chemical reactions and ingredient interactions occur as intended.
Q6: What tools are best for measuring water accurately?
A6: The best tools for measuring water accurately include liquid measuring cups for volume and kitchen scales for weight. Liquid measuring cups should be placed on a flat surface and read at eye level. Digital kitchen scales provide the most precise weight measurements.
Q7: How can I convert between cups and ounces for water?
A7: To convert between cups and ounces for water, remember that 1 cup is approximately equal to 8 fluid ounces. Therefore, you can multiply the number of cups by 8 to get the equivalent number of fluid ounces.
Q8: What are some common mistakes to avoid when measuring water?
A8: Common mistakes to avoid when measuring water include using the wrong type of measuring cup (e.g., dry measuring cup for liquids), not reading liquid measurements at eye level, and not accounting for the temperature of the water.
Q9: Does the altitude affect the weight of water?
A9: Altitude can indirectly affect the weight of water because it influences the boiling point. At higher altitudes, water boils at a lower temperature, which can affect cooking times and processes.
Q10: Where can I find reliable information about water measurements and conversions?
A10: You can find reliable information about water measurements and conversions from scientific resources, culinary guides, and reputable websites. Additionally, how.edu.vn offers expert insights and resources on accurate measurements and related topics.
15. Understanding the Metric System: Grams and Milliliters
While ounces and cups are commonly used in the United States, the metric system is prevalent in many other parts of the world. Understanding grams and milliliters is essential for international recipes and scientific applications.
15.1. Grams (Weight)
The gram (g) is a unit of mass in the metric system. It is approximately the mass of one cubic centimeter of water at its maximum density.
15.1.1. Conversion from Ounces to Grams
To convert ounces to grams, use the conversion factor: 1 ounce ≈ 28.35 grams. Therefore, 8 ounces of water (1 cup) is approximately 226.8 grams.
15.1.2. Applications in Cooking and Science
Grams are used in cooking for precise measurements, especially in baking. In scientific contexts, grams are fundamental for measuring masses in experiments and analyses.
15.2. Milliliters (Volume)
The milliliter (mL) is a unit of volume in the metric system. One milliliter is equal to one cubic centimeter (cc).
15.2.1. Conversion from Fluid Ounces to Milliliters
To convert fluid ounces to milliliters, use the conversion factor: 1 fluid ounce ≈ 29.57 milliliters. Therefore, 8 fluid ounces of water (1 cup) is approximately 236.6 milliliters.
15.2.2. Applications in Medicine and Chemistry
Milliliters are widely used in medicine for measuring dosages and in chemistry for preparing solutions.
15.3. Density Relationship
The relationship between mass, volume, and density is expressed by the formula:
Density = Mass / Volume
For water at room temperature (approximately 25°C), the density is about 1 gram per milliliter (1 g/mL).
15.4. Practical Conversions
Here are some practical conversions to keep in mind:
- 1 cup of water ≈ 227 grams
- 1 cup of water ≈ 237 milliliters
- 1 liter of water ≈ 1000 grams
- 1 liter of water ≈ 1000 milliliters
15.5. Utilizing Metric Measurements in Recipes
When using recipes that provide measurements in the metric system, ensure you have the appropriate measuring tools, such as digital scales that display grams and measuring cups or cylinders that display milliliters.
By understanding and using the metric system, you can enhance your precision in cooking, science, and other applications requiring accurate measurements.
16. Advanced Techniques: Precise Water Measurement in Scientific Research
In scientific research, the precise measurement of water is paramount for accurate and reliable results. Advanced techniques and instruments are employed to minimize errors and account for various influencing factors.
16.1. High-Precision Balances
High-precision balances, also known as analytical balances, are used to measure mass with extreme accuracy. These balances can measure down to micrograms (µg) and are essential for preparing solutions and conducting experiments where small mass differences matter.
16.1.1. Environmental Controls
To ensure accuracy, high-precision balances are typically housed in controlled environments with stable temperatures and minimal vibrations.
16.1.2. Calibration Procedures
Regular calibration with certified weights is crucial for maintaining the accuracy of these balances.
16.2. Volumetric Flasks and Pipettes
Volumetric flasks and pipettes are used for precise volume measurements. These instruments are designed to contain or deliver specific volumes with high accuracy.
16.2.1. Types of Pipettes
- Volumetric Pipettes: Designed to deliver a single, precise volume.
- Graduated Pipettes: Allow for the measurement of variable volumes.
- Micropipettes: Used to measure very small volumes (microliters).
16.2.2. Calibration of Volumetric Instruments
Volumetric flasks and pipettes are calibrated to deliver accurate volumes at specific temperatures. It’s essential to use these instruments at or near their calibration temperature.
16.3. Density Meters and Hydrometers
Density meters and hydrometers are used to measure the density of liquids. Density meters provide digital readouts of density, while hydrometers use buoyancy principles to determine density.
16.3.1. Applications in Solution Preparation
Density measurements are used to verify the concentration of solutions and to ensure accurate preparation.
16.3.2. Temperature Compensation
Many density meters have built-in temperature compensation to account for density variations due to temperature changes.
16.4. Isotope Ratio Mass Spectrometry (IRMS)
Isotope Ratio Mass Spectrometry (IRMS) is an advanced technique used to measure the isotopic composition of water. This is particularly important in environmental science and hydrology for tracing water sources and studying water cycles.
16.4.1. Measurement of Deuterium and Oxygen-18
IRMS measures the ratios of stable isotopes such as deuterium (²H) and oxygen-18 (¹⁸O) to their more abundant counterparts (¹H and ¹⁶O).
16.4.2. Applications in Hydrology
Isotopic analysis can help determine the origin and age of water samples, providing insights into groundwater flow and surface water interactions.
16.5. Error Analysis and Uncertainty Quantification
In scientific research, it is essential to quantify and report the uncertainty associated with measurements. Error analysis involves identifying potential sources of error and estimating their impact on the final results.
16.5.1. Statistical Methods
Statistical methods such as standard deviation, confidence intervals, and propagation of error are used to quantify uncertainty.
16.5.2. Reporting Measurement Uncertainty
When reporting measurement results, always include an estimate of the uncertainty to provide a complete and transparent assessment of the data.
By employing these advanced techniques and instruments, scientists can achieve highly accurate and reliable measurements of water in their research.
17. Historical Context: Evolution of Water Measurement Techniques
Understanding the historical evolution of water measurement techniques provides valuable context for appreciating modern methods and their precision.
17.1. Ancient Methods
In ancient civilizations, water measurement was crucial for irrigation, trade, and domestic use. Early methods relied on simple containers and estimations.
17.1.1. Egyptian Cubit
The Egyptian cubit, the length from the elbow to the tip of the middle finger,
