Molar mass calculation is essential for various scientific and engineering applications. Are you looking for a step-by-step guide on How To Calculate Molar Mass effectively? At HOW.EDU.VN, we provide expert insights and methodologies to simplify this process, ensuring accuracy and understanding. Mastering molar mass calculation will enhance your proficiency in chemistry and related fields, enabling you to solve complex problems with confidence. Whether you’re dealing with atomic weight, molecular weight, or formula weight, our comprehensive resources will guide you through each concept.
1. What is Molar Mass and Why is it Important?
Molar mass is defined as the mass of one mole of a substance, expressed in grams per mole (g/mol). It is a fundamental concept in chemistry, linking the macroscopic property of mass to the microscopic world of atoms and molecules. The molar mass is numerically equivalent to the atomic or molecular weight of a substance, but with units of g/mol instead of atomic mass units (amu).
1.1 Understanding Molar Mass
Molar mass, also known as molecular weight, is a crucial property used in chemistry to relate the mass of a substance to the amount of substance in moles. A mole is a unit of measurement that contains Avogadro’s number (approximately 6.022 x 10^23) of particles, which can be atoms, molecules, ions, or other entities.
1.2 Why is Molar Mass Important?
-
Stoichiometry: Molar mass is essential for stoichiometric calculations, which involve determining the quantitative relationships between reactants and products in chemical reactions. By knowing the molar masses of the substances involved, one can accurately predict the amounts of reactants needed or products formed in a reaction.
-
Solution Preparation: In preparing solutions of specific concentrations, molar mass is used to calculate the mass of solute needed to dissolve in a given volume of solvent. This is particularly important in analytical chemistry, biochemistry, and pharmacology, where precise concentrations are critical.
-
Gas Laws: Molar mass is also important in gas laws, such as the ideal gas law (PV=nRT), where n represents the number of moles. Knowing the molar mass allows one to convert between mass and moles, enabling calculations involving the volume, pressure, and temperature of gases.
-
Elemental Analysis: Molar mass is used in elemental analysis to determine the empirical formula of a compound. By measuring the mass percentages of elements in a compound, one can calculate the moles of each element and determine the simplest whole-number ratio, which represents the empirical formula.
-
Polymer Chemistry: In polymer chemistry, molar mass is a key parameter that affects the physical and mechanical properties of polymers. Techniques such as gel permeation chromatography (GPC) are used to determine the molar mass distribution of polymers, which is crucial for controlling their performance in various applications.
-
Research and Development: Molar mass determination is a routine task in many research and development laboratories. It is used to characterize new compounds, verify the identity of known compounds, and assess the purity of chemical substances.
2. Essential Tools and Resources for Molar Mass Calculation
To accurately calculate molar masses, it is important to have access to the right tools and resources. These include the periodic table, calculators, and online molar mass calculators.
2.1 The Periodic Table
The periodic table is an indispensable resource for determining the atomic masses of elements. Each element is listed with its atomic number and average atomic mass. The atomic mass is the weighted average of the masses of all the isotopes of an element and is expressed in atomic mass units (amu). For molar mass calculations, the atomic mass is used with the unit grams per mole (g/mol).
2.2 Calculators
A calculator is an essential tool for performing the arithmetic operations involved in molar mass calculations. Scientific calculators are particularly useful as they can handle exponents and other mathematical functions needed for complex calculations. Many online calculators are also available for quick and convenient calculations.
2.3 Online Molar Mass Calculators
Numerous online molar mass calculators are available, providing a convenient way to check your calculations or quickly determine the molar mass of a compound. These calculators typically require you to enter the chemical formula of the compound, and they will automatically calculate the molar mass based on the atomic masses from the periodic table. Some popular online calculators include:
- WebQC Molar Mass Calculator: A versatile calculator that can handle a wide range of chemical formulas.
- Sigma-Aldrich Molar Mass Calculator: A reliable calculator from a well-known chemical supplier.
- CalculatorSoup Molar Mass Calculator: A user-friendly calculator with a simple interface.
2.4 Chemical Databases
Chemical databases, such as PubChem and ChemSpider, provide detailed information about chemical compounds, including their molar masses, chemical structures, and other properties. These databases can be valuable resources for verifying your calculations and obtaining additional information about the compounds you are working with.
2.5 Software Tools
For more advanced calculations and data analysis, several software tools are available. These tools, such as ChemDraw and Gaussian, can perform complex calculations, simulate chemical reactions, and provide detailed information about molecular properties.
3. Step-by-Step Guide to Calculating Molar Mass
Calculating molar mass involves a systematic approach that includes identifying the chemical formula, finding the atomic masses of the elements, multiplying by the number of atoms, and summing the results.
3.1 Step 1: Identify the Chemical Formula
The first step in calculating molar mass is to identify the chemical formula of the substance. The chemical formula indicates the types and numbers of atoms present in a molecule or formula unit. For example, the chemical formula for water is H2O, indicating that each molecule of water contains two hydrogen atoms and one oxygen atom.
3.2 Step 2: Find the Atomic Masses of the Elements
Using the periodic table, find the atomic mass of each element present in the chemical formula. The atomic mass is typically found below the element symbol in the periodic table. For example, the atomic mass of hydrogen (H) is approximately 1.008 g/mol, and the atomic mass of oxygen (O) is approximately 16.00 g/mol.
3.3 Step 3: Multiply by the Number of Atoms
Multiply the atomic mass of each element by the number of atoms of that element in the chemical formula. For water (H2O), this means multiplying the atomic mass of hydrogen by 2 and the atomic mass of oxygen by 1:
- Hydrogen: 2 atoms × 1.008 g/mol = 2.016 g/mol
- Oxygen: 1 atom × 16.00 g/mol = 16.00 g/mol
3.4 Step 4: Sum the Results
Add up the results from the previous step to obtain the molar mass of the compound. For water (H2O):
- Molar mass = 2.016 g/mol (hydrogen) + 16.00 g/mol (oxygen) = 18.016 g/mol
Therefore, the molar mass of water is approximately 18.016 g/mol.
4. Examples of Molar Mass Calculations
To illustrate the process of calculating molar mass, let’s go through several examples with varying levels of complexity.
4.1 Example 1: Sodium Chloride (NaCl)
Sodium chloride (NaCl), also known as table salt, is an ionic compound composed of sodium (Na) and chlorine (Cl) atoms. To calculate its molar mass:
- Identify the Chemical Formula: NaCl
- Find the Atomic Masses:
- Sodium (Na): 22.99 g/mol
- Chlorine (Cl): 35.45 g/mol
- Multiply by the Number of Atoms:
- Sodium: 1 atom × 22.99 g/mol = 22.99 g/mol
- Chlorine: 1 atom × 35.45 g/mol = 35.45 g/mol
- Sum the Results:
- Molar mass = 22.99 g/mol (sodium) + 35.45 g/mol (chlorine) = 58.44 g/mol
Therefore, the molar mass of sodium chloride is approximately 58.44 g/mol.
4.2 Example 2: Glucose (C6H12O6)
Glucose (C6H12O6) is a simple sugar molecule that serves as an important energy source for living organisms. To calculate its molar mass:
- Identify the Chemical Formula: C6H12O6
- Find the Atomic Masses:
- Carbon (C): 12.01 g/mol
- Hydrogen (H): 1.008 g/mol
- Oxygen (O): 16.00 g/mol
- Multiply by the Number of Atoms:
- Carbon: 6 atoms × 12.01 g/mol = 72.06 g/mol
- Hydrogen: 12 atoms × 1.008 g/mol = 12.096 g/mol
- Oxygen: 6 atoms × 16.00 g/mol = 96.00 g/mol
- Sum the Results:
- Molar mass = 72.06 g/mol (carbon) + 12.096 g/mol (hydrogen) + 96.00 g/mol (oxygen) = 180.156 g/mol
Therefore, the molar mass of glucose is approximately 180.156 g/mol.
4.3 Example 3: Copper(II) Sulfate Pentahydrate (CuSO4·5H2O)
Copper(II) sulfate pentahydrate (CuSO4·5H2O) is a hydrated salt, meaning it contains water molecules incorporated into its crystal structure. To calculate its molar mass:
- Identify the Chemical Formula: CuSO4·5H2O
- Find the Atomic Masses:
- Copper (Cu): 63.55 g/mol
- Sulfur (S): 32.07 g/mol
- Oxygen (O): 16.00 g/mol
- Hydrogen (H): 1.008 g/mol
- Multiply by the Number of Atoms:
- Copper: 1 atom × 63.55 g/mol = 63.55 g/mol
- Sulfur: 1 atom × 32.07 g/mol = 32.07 g/mol
- Oxygen: 4 atoms (from CuSO4) + 5 atoms (from 5H2O) × 16.00 g/mol = 9 × 16.00 g/mol = 144.00 g/mol
- Hydrogen: 10 atoms (from 5H2O) × 1.008 g/mol = 10.08 g/mol
- Sum the Results:
- Molar mass = 63.55 g/mol (copper) + 32.07 g/mol (sulfur) + 144.00 g/mol (oxygen) + 10.08 g/mol (hydrogen) = 249.70 g/mol
Therefore, the molar mass of copper(II) sulfate pentahydrate is approximately 249.70 g/mol.
4.4 Example 4: Potassium Permanganate (KMnO4)
Potassium permanganate (KMnO4) is an inorganic compound used in various chemical applications. To calculate its molar mass:
- Identify the Chemical Formula: KMnO4
- Find the Atomic Masses:
- Potassium (K): 39.10 g/mol
- Manganese (Mn): 54.94 g/mol
- Oxygen (O): 16.00 g/mol
- Multiply by the Number of Atoms:
- Potassium: 1 atom × 39.10 g/mol = 39.10 g/mol
- Manganese: 1 atom × 54.94 g/mol = 54.94 g/mol
- Oxygen: 4 atoms × 16.00 g/mol = 64.00 g/mol
- Sum the Results:
- Molar mass = 39.10 g/mol (potassium) + 54.94 g/mol (manganese) + 64.00 g/mol (oxygen) = 158.04 g/mol
Therefore, the molar mass of potassium permanganate is approximately 158.04 g/mol.
4.5 Example 5: Ammonium Sulfate ((NH4)2SO4)
Ammonium sulfate ((NH4)2SO4) is an inorganic salt used as a fertilizer. To calculate its molar mass:
- Identify the Chemical Formula: (NH4)2SO4
- Find the Atomic Masses:
- Nitrogen (N): 14.01 g/mol
- Hydrogen (H): 1.008 g/mol
- Sulfur (S): 32.07 g/mol
- Oxygen (O): 16.00 g/mol
- Multiply by the Number of Atoms:
- Nitrogen: 2 atoms × 14.01 g/mol = 28.02 g/mol
- Hydrogen: 8 atoms × 1.008 g/mol = 8.064 g/mol
- Sulfur: 1 atom × 32.07 g/mol = 32.07 g/mol
- Oxygen: 4 atoms × 16.00 g/mol = 64.00 g/mol
- Sum the Results:
- Molar mass = 28.02 g/mol (nitrogen) + 8.064 g/mol (hydrogen) + 32.07 g/mol (sulfur) + 64.00 g/mol (oxygen) = 132.154 g/mol
Therefore, the molar mass of ammonium sulfate is approximately 132.154 g/mol.
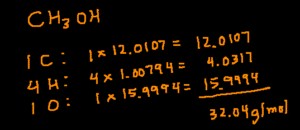 Periodic Table Showing Atomic Masses
Periodic Table Showing Atomic Masses
5. Common Mistakes to Avoid When Calculating Molar Mass
Calculating molar mass is a straightforward process, but it is important to avoid common mistakes that can lead to inaccurate results.
5.1 Incorrectly Identifying the Chemical Formula
One of the most common mistakes is incorrectly identifying the chemical formula of the compound. Ensure that you have the correct formula before proceeding with the calculation. Double-check subscripts and parentheses to avoid errors.
5.2 Using Incorrect Atomic Masses
Using incorrect atomic masses from the periodic table is another common mistake. Always use the most accurate and up-to-date values for atomic masses. Refer to a reliable periodic table or online database to ensure accuracy.
5.3 Not Multiplying by the Number of Atoms
Forgetting to multiply the atomic mass of each element by the number of atoms in the chemical formula is a frequent error. Ensure that you account for all atoms present in the compound, especially when dealing with complex formulas or compounds with polyatomic ions.
5.4 Errors in Arithmetic
Simple arithmetic errors can also lead to incorrect molar mass calculations. Take your time and double-check your calculations to avoid mistakes in addition, multiplication, or division.
5.5 Ignoring Hydrates
When calculating the molar mass of hydrates, it is important to include the mass of the water molecules associated with the compound. Failing to account for the water molecules will result in an underestimation of the molar mass.
6. Advanced Techniques in Molar Mass Determination
While calculating molar mass is generally straightforward, advanced techniques are used in certain situations to determine molar masses with greater accuracy or for complex substances.
6.1 Mass Spectrometry
Mass spectrometry is a powerful analytical technique used to determine the molar mass of a compound with high precision. In mass spectrometry, a sample is ionized, and the ions are separated based on their mass-to-charge ratio. By measuring the mass-to-charge ratio of the ions, the molar mass of the compound can be accurately determined. Mass spectrometry is particularly useful for analyzing complex mixtures of compounds and for identifying unknown substances.
6.2 Gel Permeation Chromatography (GPC)
Gel permeation chromatography (GPC), also known as size exclusion chromatography (SEC), is a technique used to determine the molar mass distribution of polymers. In GPC, a polymer sample is dissolved in a solvent and passed through a column packed with a porous gel material. The polymer molecules are separated based on their size, with larger molecules eluting from the column first. By calibrating the column with standards of known molar mass, the molar mass distribution of the polymer can be determined.
6.3 Osmometry
Osmometry is a technique used to determine the number-average molar mass of a polymer. In osmometry, the osmotic pressure of a polymer solution is measured. The osmotic pressure is related to the number of polymer molecules in the solution, and by measuring the osmotic pressure, the number-average molar mass can be determined.
6.4 Light Scattering
Light scattering is a technique used to determine the weight-average molar mass of a polymer. In light scattering, a beam of light is passed through a polymer solution, and the intensity of the scattered light is measured. The intensity of the scattered light is related to the size and concentration of the polymer molecules, and by measuring the scattered light, the weight-average molar mass can be determined.
7. Real-World Applications of Molar Mass
Molar mass is a fundamental concept with numerous applications in various fields, including chemistry, biology, engineering, and medicine.
7.1 Chemical Synthesis
In chemical synthesis, molar mass is used to calculate the amounts of reactants needed to synthesize a desired product. By knowing the molar masses of the reactants and products, chemists can accurately weigh out the required amounts of each substance to ensure that the reaction proceeds efficiently and produces the maximum yield of the desired product.
7.2 Pharmaceutical Industry
In the pharmaceutical industry, molar mass is used to determine the correct dosage of drugs. The dosage of a drug is typically expressed in terms of milligrams per kilogram of body weight, and by knowing the molar mass of the drug, pharmacists can calculate the correct amount of drug to administer to a patient.
7.3 Environmental Science
In environmental science, molar mass is used to calculate the concentration of pollutants in air and water samples. By measuring the mass of a pollutant in a known volume of air or water, scientists can calculate the concentration of the pollutant in terms of moles per liter or parts per million.
7.4 Food Science
In food science, molar mass is used to determine the nutritional content of foods. By knowing the molar masses of the various components of a food, such as carbohydrates, proteins, and fats, food scientists can calculate the caloric content and nutritional value of the food.
7.5 Materials Science
In materials science, molar mass is used to characterize the properties of materials. For example, the molar mass of a polymer can affect its strength, elasticity, and thermal stability. By controlling the molar mass of a polymer, materials scientists can tailor its properties to meet the requirements of specific applications.
8. The Role of Molar Mass in Stoichiometry
Stoichiometry is the study of the quantitative relationships between reactants and products in chemical reactions. Molar mass plays a central role in stoichiometric calculations, allowing chemists to convert between mass, moles, and number of particles.
8.1 Mole Ratios
In a balanced chemical equation, the coefficients in front of the chemical formulas represent the mole ratios of the reactants and products. For example, in the balanced equation:
2H2 + O2 → 2H2O
The mole ratio of hydrogen (H2) to oxygen (O2) is 2:1, and the mole ratio of hydrogen to water (H2O) is 2:2 or 1:1. These mole ratios can be used to calculate the amount of one substance needed to react with a given amount of another substance.
8.2 Limiting Reactant
In many chemical reactions, one of the reactants is present in excess, while the other reactant is the limiting reactant. The limiting reactant is the reactant that is completely consumed in the reaction, and it determines the maximum amount of product that can be formed. To identify the limiting reactant, one must calculate the number of moles of each reactant and compare them to the mole ratios in the balanced equation.
8.3 Theoretical Yield
The theoretical yield is the maximum amount of product that can be formed in a chemical reaction, assuming that all of the limiting reactant is converted to product. The theoretical yield is calculated using the mole ratios in the balanced equation and the molar masses of the reactants and products.
8.4 Percent Yield
The percent yield is the actual yield of product obtained in a chemical reaction, expressed as a percentage of the theoretical yield. The percent yield is a measure of the efficiency of the reaction, and it can be affected by factors such as incomplete reactions, side reactions, and loss of product during purification.
9. Tips and Tricks for Mastering Molar Mass Calculations
To become proficient in molar mass calculations, it is important to practice regularly and develop a systematic approach. Here are some tips and tricks to help you master this skill:
9.1 Practice Regularly
The more you practice molar mass calculations, the more comfortable and confident you will become. Work through a variety of examples, starting with simple compounds and progressing to more complex ones.
9.2 Use a Systematic Approach
Develop a systematic approach to molar mass calculations, following the steps outlined in this guide. This will help you avoid mistakes and ensure that you are accounting for all atoms present in the compound.
9.3 Double-Check Your Work
Always double-check your calculations to ensure that you have not made any arithmetic errors or overlooked any atoms. It can be helpful to have a friend or colleague review your work as well.
9.4 Use Online Resources
Take advantage of the numerous online resources available for molar mass calculations, such as calculators, databases, and tutorials. These resources can help you check your work, learn new techniques, and deepen your understanding of the concept.
9.5 Understand the Underlying Principles
Focus on understanding the underlying principles of molar mass calculations, rather than simply memorizing formulas and procedures. This will help you apply the concept to new and unfamiliar situations.
10. How HOW.EDU.VN Can Help You Master Molar Mass Calculations
At HOW.EDU.VN, we understand the challenges students and professionals face when dealing with complex chemical concepts like molar mass calculation. That’s why we offer a unique platform that connects you with top-tier Ph.D. experts who can provide personalized guidance and support.
10.1 Connect with Expert Ph.D. Tutors
Our platform features a network of over 100 Ph.D. experts from various fields, including chemistry, ready to assist you with molar mass calculations and related topics. These experts bring years of experience and in-depth knowledge to help you understand the fundamental principles and apply them effectively.
10.2 Personalized Guidance and Support
We provide personalized guidance tailored to your specific needs. Whether you’re struggling with basic concepts or need help with advanced techniques, our experts can offer one-on-one tutoring sessions to address your questions and concerns.
10.3 Step-by-Step Problem Solving
Our experts guide you through the process of solving molar mass calculation problems step-by-step. They break down complex problems into manageable steps, making it easier for you to understand and apply the concepts.
10.4 Access to Comprehensive Resources
Gain access to a wealth of comprehensive resources, including tutorials, practice problems, and real-world examples. These resources are designed to reinforce your understanding of molar mass calculations and help you build confidence in your abilities.
10.5 Flexible Learning Options
We offer flexible learning options to fit your schedule and learning preferences. Whether you prefer online tutoring sessions, email support, or access to our extensive library of resources, we have a solution that works for you.
11. Frequently Asked Questions (FAQ) About Molar Mass
11.1 What is the difference between atomic mass and molar mass?
Atomic mass is the mass of a single atom, expressed in atomic mass units (amu), while molar mass is the mass of one mole of a substance, expressed in grams per mole (g/mol). The molar mass is numerically equal to the atomic mass, but with different units.
11.2 How do I find the atomic mass of an element?
You can find the atomic mass of an element on the periodic table. The atomic mass is typically located below the element symbol and is expressed in atomic mass units (amu).
11.3 What is a mole?
A mole is a unit of measurement that contains Avogadro’s number (approximately 6.022 x 10^23) of particles, which can be atoms, molecules, ions, or other entities.
11.4 How do I calculate the molar mass of a compound?
To calculate the molar mass of a compound, identify the chemical formula, find the atomic masses of the elements, multiply by the number of atoms, and sum the results.
11.5 What is the molar mass of water (H2O)?
The molar mass of water (H2O) is approximately 18.016 g/mol.
11.6 How do I calculate the molar mass of a hydrate?
To calculate the molar mass of a hydrate, include the mass of the water molecules associated with the compound in addition to the mass of the anhydrous salt.
11.7 What is stoichiometry?
Stoichiometry is the study of the quantitative relationships between reactants and products in chemical reactions.
11.8 How is molar mass used in stoichiometry?
Molar mass is used in stoichiometry to convert between mass, moles, and number of particles, allowing chemists to calculate the amounts of reactants needed or products formed in a chemical reaction.
11.9 What is the limiting reactant?
The limiting reactant is the reactant that is completely consumed in a chemical reaction, and it determines the maximum amount of product that can be formed.
11.10 How can HOW.EDU.VN help me with molar mass calculations?
HOW.EDU.VN connects you with expert Ph.D. tutors who can provide personalized guidance and support, step-by-step problem-solving, and access to comprehensive resources to help you master molar mass calculations.
12. Take the Next Step: Connect with a Ph.D. Expert Today
Calculating molar mass doesn’t have to be a daunting task. With the right guidance and resources, you can master this essential skill and excel in your studies or professional endeavors. At HOW.EDU.VN, we are committed to providing you with the support you need to succeed.
Ready to Transform Your Understanding of Molar Mass?
Don’t let the complexities of molar mass calculations hold you back. Contact HOW.EDU.VN today and discover how our team of expert Ph.D. tutors can help you achieve your goals. Whether you need help with basic concepts, advanced techniques, or anything in between, we have a solution that works for you.
- Connect with over 100 Ph.D. experts: Gain access to a diverse network of knowledgeable professionals who can provide personalized guidance and support.
- Get personalized guidance and support: Receive tailored assistance to address your specific needs and learning style.
- Master step-by-step problem solving: Learn how to break down complex problems into manageable steps and apply the concepts effectively.
- Access comprehensive resources: Take advantage of our extensive library of tutorials, practice problems, and real-world examples.
- Enjoy flexible learning options: Choose a learning format that fits your schedule and preferences.
Contact Us Today
Don’t wait any longer to unlock your full potential. Contact HOW.EDU.VN today and take the next step towards mastering molar mass calculations.
Address: 456 Expertise Plaza, Consult City, CA 90210, United States
WhatsApp: +1 (310) 555-1212
Website: HOW.EDU.VN
Let how.edu.vn be your partner in success. Our team of expert Ph.D. tutors is ready to help you conquer your academic and professional challenges. Connect with us today and experience the difference that personalized guidance can make.