Finding oxidation numbers can seem daunting, but HOW.EDU.VN makes it simple. This guide provides a clear method on oxidation state assignment, oxidation number calculation and understanding redox reactions. Discover expert advice and resources for mastering this fundamental concept.
1. Understanding Oxidation Number: The Basics
The oxidation number, also known as oxidation state, signifies the hypothetical charge an atom would have if all bonds were completely ionic. It’s a concept crucial for understanding redox reactions and the behavior of elements in compounds. But what exactly does it represent, and why is it so important? The oxidation number reflects the degree of oxidation (loss of electrons) of an atom in a chemical compound. It can be positive, negative, or zero, indicating the number of electrons an atom has gained, lost, or shared when forming chemical bonds. As explained in “Chemistry: The Molecular Nature of Matter and Change” by Silberberg and Amateis, understanding oxidation numbers helps predict the behavior of elements during chemical reactions and aids in balancing complex equations. This fundamental understanding is vital for students, researchers, and professionals in chemistry.
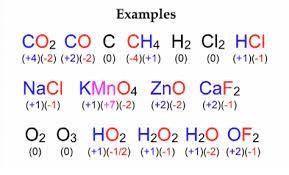 Oxidation State Examples
Oxidation State Examples
1.1. Defining Oxidation Number and Oxidation State
Oxidation number and oxidation state are often used interchangeably, but understanding their nuances is essential. The oxidation number is a numerical representation of an atom’s oxidation state, while the oxidation state describes the degree of oxidation. The International Union of Pure and Applied Chemistry (IUPAC) defines oxidation state as “the charge of this atom after ionic approximation of its heteronuclear bonds.” This means that the oxidation state is what you would expect the charge to be if the bonds were ionic. In most cases, the oxidation number is simply the numerical value of the oxidation state. According to “Inorganic Chemistry” by Shriver and Atkins, while these terms are often used synonymously, it’s important to remember that oxidation state is a theoretical concept based on idealized ionic bonding.
1.2. Why are Oxidation Numbers Important?
Oxidation numbers are a cornerstone of chemistry for several reasons. They allow us to:
- Identify Redox Reactions: Oxidation numbers are essential for recognizing oxidation-reduction (redox) reactions, where electrons are transferred between atoms.
- Balance Chemical Equations: Assigning oxidation numbers is crucial for balancing complex redox reactions, ensuring the conservation of mass and charge.
- Predict Chemical Behavior: Understanding oxidation states helps predict how elements will behave in chemical reactions, guiding the design of experiments and synthesis of new compounds.
- Name Chemical Compounds: The oxidation state is often included in the names of chemical compounds, particularly those involving transition metals, to specify the charge of the metal ion.
- Understand Electrochemical Processes: Oxidation numbers are fundamental to understanding electrochemical cells, batteries, and corrosion processes.
- Analyze Molecular Structures: Oxidation states can offer insights into the electronic structure and bonding within molecules.
1.3. Common Oxidation States of Elements
Many elements exhibit common oxidation states, which are helpful to memorize. Here’s a brief overview:
| Element Group | Common Oxidation States | Examples |
|---|---|---|
| Alkali Metals (Group 1) | +1 | Na+, K+, Li+ |
| Alkaline Earth Metals (Group 2) | +2 | Mg2+, Ca2+, Ba2+ |
| Halogens (Group 17) | -1 | Cl-, Br-, I- |
| Oxygen | -2 | H2O, CO2 (except in peroxides) |
| Hydrogen | +1 | H2O, HCl (except in metal hydrides) |
These common oxidation states can serve as a starting point when determining the oxidation numbers of atoms in more complex compounds. However, it is important to know the rules for assigning oxidation numbers, which are discussed in the next section.
2. Rules for Assigning Oxidation Numbers
Assigning oxidation numbers follows a specific set of rules. Mastering these rules is essential for accurately determining the oxidation state of atoms in any compound or ion. These rules are based on electronegativity differences and common chemical behaviors.
2.1. Rule 1: Elements in Their Elemental State
The oxidation number of an atom in its elemental state is always zero. This applies to all elements, whether they exist as monatomic species (e.g., He, Ar) or as molecules (e.g., H2, O2, S8). For instance, in a sample of pure iron (Fe), the oxidation number of each iron atom is 0. Similarly, in a molecule of diatomic chlorine (Cl2), each chlorine atom has an oxidation number of 0. This rule is fundamental because it establishes a baseline for comparing the oxidation states of elements in compounds.
2.2. Rule 2: Monoatomic Ions
The oxidation number of a monoatomic ion is equal to its charge. For example, the sodium ion (Na+) has an oxidation number of +1, and the chloride ion (Cl-) has an oxidation number of -1. This rule is straightforward and directly reflects the number of electrons gained or lost by the atom in forming the ion. In the case of complex ions, the overall charge of the ion must equal the sum of the oxidation numbers of all its atoms.
2.3. Rule 3: Fluorine
Fluorine is always assigned an oxidation number of -1 in its compounds. Fluorine is the most electronegative element, meaning it has the strongest tendency to attract electrons. Therefore, it will always have a negative oxidation state when bonded to other elements. For example, in hydrogen fluoride (HF), fluorine has an oxidation number of -1, and hydrogen has an oxidation number of +1.
2.4. Rule 4: Oxygen
Oxygen usually has an oxidation number of -2 in its compounds. This is because oxygen is highly electronegative, second only to fluorine. However, there are some exceptions to this rule:
- With Fluorine (OF2): When combined with fluorine, oxygen has a positive oxidation number because fluorine is more electronegative. In OF2, oxygen has an oxidation number of +2.
- In Peroxides (H2O2, Na2O2): In peroxides, oxygen has an oxidation number of -1. Peroxides contain the O-O bond, and the presence of this bond affects the oxidation state of oxygen.
- In Superoxides (KO2): In superoxides, oxygen has an oxidation number of -1/2. Superoxides contain the O2- ion.
2.5. Rule 5: Hydrogen
Hydrogen typically has an oxidation number of +1 in its compounds. This is because hydrogen is generally less electronegative than the elements it bonds with. However, there is an exception:
- In Metal Hydrides (NaH, LiH): When combined with metals, hydrogen has an oxidation number of -1. In metal hydrides, hydrogen is more electronegative than the metal, so it gains electrons and becomes negatively charged.
2.6. Rule 6: Alkali Metals
Alkali metals (Group 1 elements) always have an oxidation number of +1 in their compounds. These elements readily lose one electron to form stable ions with a +1 charge. Examples include sodium (Na), potassium (K), and lithium (Li).
2.7. Rule 7: Alkaline Earth Metals
Alkaline earth metals (Group 2 elements) always have an oxidation number of +2 in their compounds. These elements readily lose two electrons to form stable ions with a +2 charge. Examples include magnesium (Mg), calcium (Ca), and barium (Ba).
2.8. Rule 8: Halogens (Except Fluorine)
Halogens (Group 17 elements), except for fluorine, usually have an oxidation number of -1 in binary compounds (compounds with only two elements). However, when combined with oxygen or other more electronegative halogens, they can have positive oxidation numbers. For example, in sodium chloride (NaCl), chlorine has an oxidation number of -1. But in perchloric acid (HClO4), chlorine has an oxidation number of +7.
2.9. Rule 9: Sum of Oxidation Numbers in a Neutral Compound
The sum of the oxidation numbers of all atoms in a neutral compound must equal zero. This rule is based on the principle of charge neutrality. For example, in water (H2O), the oxidation number of each hydrogen atom is +1, and the oxidation number of the oxygen atom is -2. The sum of the oxidation numbers is (2 x +1) + (-2) = 0.
2.10. Rule 10: Sum of Oxidation Numbers in a Polyatomic Ion
The sum of the oxidation numbers of all atoms in a polyatomic ion must equal the charge of the ion. For example, in the sulfate ion (SO42-), the oxidation number of each oxygen atom is -2. The sum of the oxidation numbers of the four oxygen atoms is (4 x -2) = -8. Since the overall charge of the sulfate ion is -2, the oxidation number of the sulfur atom must be +6 to satisfy the equation: (+6) + (-8) = -2.
These rules provide a systematic approach for assigning oxidation numbers. By applying these rules in order, you can determine the oxidation state of each atom in a compound or ion, no matter how complex. Remember to always double-check your work and ensure that the sum of the oxidation numbers matches the overall charge of the species.
3. Step-by-Step Guide to Finding Oxidation Numbers
Now that you understand the rules, let’s walk through a step-by-step guide to finding oxidation numbers in various compounds and ions. This practical approach will solidify your understanding and enable you to tackle any problem with confidence.
3.1. Step 1: Identify the Compound or Ion
The first step is to clearly identify the chemical formula of the compound or ion you are working with. This will provide you with the necessary information about the types and numbers of atoms present. For example, you might be asked to find the oxidation numbers in potassium permanganate (KMnO4) or the ammonium ion (NH4+).
3.2. Step 2: Apply the Rules in Order
Apply the oxidation number rules in the order they are presented in Section 2. Start with the rules that are most definitive and work your way down. This systematic approach will help you avoid errors and ensure that you consider all relevant factors.
- Elemental State: If the compound contains any elements in their elemental state, assign them an oxidation number of 0.
- Monoatomic Ions: If the compound contains any monoatomic ions, assign them an oxidation number equal to their charge.
- Fluorine: If the compound contains fluorine, assign it an oxidation number of -1.
- Oxygen: If the compound contains oxygen, assign it an oxidation number of -2, unless it is combined with fluorine or in a peroxide.
- Hydrogen: If the compound contains hydrogen, assign it an oxidation number of +1, unless it is combined with a metal.
- Alkali Metals: If the compound contains alkali metals, assign them an oxidation number of +1.
- Alkaline Earth Metals: If the compound contains alkaline earth metals, assign them an oxidation number of +2.
- Halogens (Except Fluorine): If the compound contains halogens (except fluorine) in a binary compound, assign them an oxidation number of -1.
3.3. Step 3: Calculate the Unknown Oxidation Number
After applying the known rules, you may have one or more atoms with unknown oxidation numbers. Use the rules regarding the sum of oxidation numbers to calculate the unknown values. Remember that the sum of the oxidation numbers in a neutral compound must equal zero, and the sum of the oxidation numbers in a polyatomic ion must equal the charge of the ion.
3.4. Step 4: Double-Check Your Work
Always double-check your work to ensure that the sum of the oxidation numbers matches the overall charge of the species. This will help you catch any errors and ensure that your answer is correct.
4. Examples of Finding Oxidation Numbers
Let’s apply the step-by-step guide to several examples to illustrate the process of finding oxidation numbers. These examples cover a range of compounds and ions, allowing you to practice and solidify your skills.
4.1. Example 1: Water (H2O)
- Identify the Compound: The compound is water (H2O).
- Apply the Rules:
- Hydrogen (Rule 5): Hydrogen is usually +1.
- Oxygen (Rule 4): Oxygen is usually -2.
- Calculate the Unknown:
- The sum of the oxidation numbers must equal zero (Rule 9).
- (2 x +1) + (-2) = 0
- Double-Check:
- The sum of the oxidation numbers is zero, which matches the overall charge of the compound.
- Therefore, the oxidation number of hydrogen is +1, and the oxidation number of oxygen is -2.
4.2. Example 2: Potassium Permanganate (KMnO4)
- Identify the Compound: The compound is potassium permanganate (KMnO4).
- Apply the Rules:
- Potassium (Rule 6): Potassium is always +1.
- Oxygen (Rule 4): Oxygen is usually -2.
- Calculate the Unknown:
- The sum of the oxidation numbers must equal zero (Rule 9).
- (+1) + (x) + (4 x -2) = 0, where x is the oxidation number of manganese (Mn).
- +1 + x – 8 = 0
- x = +7
- Double-Check:
- The sum of the oxidation numbers is zero, which matches the overall charge of the compound.
- Therefore, the oxidation number of potassium is +1, the oxidation number of manganese is +7, and the oxidation number of oxygen is -2.
4.3. Example 3: Sulfate Ion (SO42-)
- Identify the Ion: The ion is the sulfate ion (SO42-).
- Apply the Rules:
- Oxygen (Rule 4): Oxygen is usually -2.
- Calculate the Unknown:
- The sum of the oxidation numbers must equal the charge of the ion (Rule 10).
- (x) + (4 x -2) = -2, where x is the oxidation number of sulfur (S).
- x – 8 = -2
- x = +6
- Double-Check:
- The sum of the oxidation numbers is -2, which matches the overall charge of the ion.
- Therefore, the oxidation number of sulfur is +6, and the oxidation number of oxygen is -2.
4.4. Example 4: Dichromate Ion (Cr2O72-)
- Identify the Ion: The ion is the dichromate ion (Cr2O72-).
- Apply the Rules:
- Oxygen (Rule 4): Oxygen is usually -2.
- Calculate the Unknown:
- The sum of the oxidation numbers must equal the charge of the ion (Rule 10).
- (2x) + (7 x -2) = -2, where x is the oxidation number of chromium (Cr).
- 2x – 14 = -2
- 2x = +12
- x = +6
- Double-Check:
- The sum of the oxidation numbers is -2, which matches the overall charge of the ion.
- Therefore, the oxidation number of chromium is +6, and the oxidation number of oxygen is -2.
By working through these examples, you can see how the oxidation number rules are applied in practice. Remember to follow the step-by-step guide and double-check your work to ensure accuracy.
5. Common Mistakes and How to Avoid Them
Even with a solid understanding of the rules, it’s easy to make mistakes when assigning oxidation numbers. Here are some common pitfalls and strategies to avoid them:
5.1. Forgetting the Order of Rules
One of the most common mistakes is not applying the rules in the correct order. Always start with the most definitive rules, such as those for elements in their elemental state or monoatomic ions, and work your way down. This will prevent you from making incorrect assumptions and assigning incorrect oxidation numbers.
5.2. Ignoring Exceptions to Rules
Many rules have exceptions, such as the oxidation number of oxygen in peroxides or hydrogen in metal hydrides. Failing to recognize these exceptions can lead to incorrect answers. Pay close attention to the specific compounds or ions you are working with and be aware of any potential exceptions to the rules.
5.3. Not Considering the Overall Charge
It’s crucial to remember that the sum of the oxidation numbers in a neutral compound must equal zero, and the sum of the oxidation numbers in a polyatomic ion must equal the charge of the ion. Neglecting to consider the overall charge can result in incorrect calculations. Always double-check that your final answer satisfies the charge requirement.
5.4. Misinterpreting Chemical Formulas
Misinterpreting chemical formulas can lead to errors in assigning oxidation numbers. Make sure you correctly identify the types and numbers of atoms present in the compound or ion. Pay attention to subscripts and parentheses, as they indicate the number of atoms or groups of atoms present in the formula.
5.5. Not Double-Checking Your Work
Failing to double-check your work is a common mistake that can easily be avoided. Always take the time to review your calculations and ensure that the sum of the oxidation numbers matches the overall charge of the species. This simple step can help you catch any errors and ensure that your answer is correct.
5.6. Mixing Up Oxidation Number and Charge
While related, oxidation number and charge are not the same thing. The oxidation number is a theoretical concept based on idealized ionic bonding, while the charge is the actual electrical charge of an ion or atom. Avoid using these terms interchangeably and understand the distinction between them.
By being aware of these common mistakes and taking steps to avoid them, you can improve your accuracy and confidence in assigning oxidation numbers. Remember to practice regularly and seek help when needed.
6. Applications of Oxidation Numbers
Oxidation numbers are not just theoretical constructs; they have numerous practical applications in chemistry and related fields. Understanding oxidation numbers is essential for:
6.1. Balancing Redox Reactions
Redox reactions are fundamental to many chemical processes, including combustion, corrosion, and respiration. Balancing redox reactions involves ensuring that the number of electrons lost in the oxidation half-reaction equals the number of electrons gained in the reduction half-reaction. Oxidation numbers are used to track the transfer of electrons and balance the equations.
6.2. Identifying Oxidizing and Reducing Agents
In a redox reaction, the oxidizing agent is the substance that gains electrons (is reduced), and the reducing agent is the substance that loses electrons (is oxidized). Oxidation numbers are used to identify the oxidizing and reducing agents by observing the change in oxidation state of the atoms involved in the reaction.
6.3. Predicting Reaction Products
Knowing the oxidation states of reactants can help predict the products of a chemical reaction. By understanding how oxidation states change during the reaction, you can determine the most likely products and write balanced chemical equations.
6.4. Understanding Electrochemical Cells
Electrochemical cells, such as batteries and fuel cells, rely on redox reactions to generate electrical energy. Oxidation numbers are used to understand the electron flow and predict the voltage and current of the cell.
6.5. Corrosion Prevention
Corrosion is a redox process that causes the degradation of metals. Understanding oxidation numbers helps in developing strategies to prevent corrosion, such as using protective coatings or sacrificial anodes.
6.6. Environmental Chemistry
Oxidation numbers are used to study the fate of pollutants in the environment. For example, understanding the oxidation states of metals in soil and water can help in developing remediation strategies.
6.7. Organic Chemistry
Oxidation numbers can be used to track oxidation and reduction reactions in organic chemistry. This is particularly useful in understanding the mechanisms of organic reactions.
7. Advanced Concepts Related to Oxidation Numbers
While the basic rules for assigning oxidation numbers are straightforward, there are some advanced concepts that build upon this foundation. Exploring these concepts will deepen your understanding of oxidation numbers and their applications.
7.1. Fractional Oxidation Numbers
In some compounds, the average oxidation number of an element may be a fraction. This occurs when the element exists in multiple oxidation states within the same compound. For example, in the mixed-valence oxide Fe3O4, iron exists in both the +2 and +3 oxidation states. The average oxidation number of iron in Fe3O4 is +8/3.
7.2. Oxidation Numbers in Organic Compounds
Assigning oxidation numbers in organic compounds can be more challenging than in inorganic compounds due to the presence of covalent bonds and complex structures. However, the same basic principles apply. Carbon is assigned an oxidation number based on the electronegativity of the atoms it is bonded to. For example, in methane (CH4), carbon has an oxidation number of -4, while in carbon dioxide (CO2), carbon has an oxidation number of +4.
7.3. Formal Charge vs. Oxidation Number
Formal charge and oxidation number are both used to describe the distribution of electrons in a molecule, but they are calculated differently and represent different concepts. Formal charge assumes that electrons are shared equally between atoms, while oxidation number assumes that electrons are transferred completely to the more electronegative atom. Formal charge is useful for determining the most stable Lewis structure of a molecule, while oxidation number is useful for tracking electron transfer in redox reactions.
7.4. Limitations of Oxidation Numbers
Oxidation numbers are a useful tool, but they have limitations. They are based on a simplified model of ionic bonding and do not always accurately reflect the true distribution of electrons in a molecule. In some cases, oxidation numbers can be ambiguous or misleading. It’s important to remember that oxidation numbers are a theoretical construct and should be used with caution.
8. How HOW.EDU.VN Can Help You Master Oxidation Numbers
At HOW.EDU.VN, we understand the challenges students face when learning chemistry. That’s why we offer a range of resources to help you master oxidation numbers and other key concepts.
8.1. Expertly Crafted Content
Our website features expertly crafted articles, tutorials, and videos that explain oxidation numbers in a clear and concise manner. Our content is designed to be accessible to students of all levels, from beginners to advanced learners.
8.2. Personalized Learning Experience
We offer personalized learning experiences tailored to your individual needs. Our adaptive learning platform assesses your knowledge and provides targeted feedback and recommendations.
8.3. Access to Expert Tutors
Connect with our team of experienced chemistry tutors who can provide one-on-one support and answer your questions. Our tutors are available 24/7 to help you with any chemistry topic.
8.4. Practice Problems and Quizzes
Test your knowledge with our extensive collection of practice problems and quizzes. Our problems are designed to challenge you and help you develop your problem-solving skills.
8.5. Community Forum
Join our community forum to connect with other students and share your knowledge. Our forum is a great place to ask questions, get help with homework, and discuss chemistry topics.
8.6. Advanced Tools and Resources
Access advanced tools and resources, including calculators, periodic tables, and chemical databases. Our tools are designed to help you with your chemistry studies and research.
9. Testimonials and Success Stories
Don’t just take our word for it. Here are some testimonials from students who have used HOW.EDU.VN to master oxidation numbers and other chemistry concepts:
- “HOW.EDU.VN helped me understand oxidation numbers in a way that no other resource could. The step-by-step guide and practice problems were invaluable.” – Sarah, High School Student
- “I was struggling with redox reactions until I found HOW.EDU.VN. The expertly crafted content and access to expert tutors made all the difference.” – Michael, College Student
- “HOW.EDU.VN is the best resource for chemistry I’ve ever used. The personalized learning experience and community forum are amazing.” – Emily, Graduate Student
These success stories demonstrate the effectiveness of HOW.EDU.VN in helping students master oxidation numbers and other chemistry concepts.
10. Call to Action: Get Expert Chemistry Help Now
Are you struggling with oxidation numbers or other chemistry concepts? Don’t waste any more time trying to figure it out on your own. Contact HOW.EDU.VN today and get expert help from our team of experienced chemistry tutors.
10.1. Connect with Experienced Chemistry Experts
Our team of over 100 renowned PhDs are available to provide personalized guidance and support. Whether you’re tackling complex chemical equations or need clarification on oxidation states, our experts offer tailored solutions.
10.2. Get Personalized Solutions
We understand that every student learns differently. That’s why we offer personalized learning experiences tailored to your individual needs.
10.3. Save Time and Improve Your Grades
Don’t waste any more time struggling with chemistry. Contact HOW.EDU.VN today and get the help you need to succeed.
10.4. Ensure Confidential and Reliable Guidance
Your academic success is our priority. We uphold the highest standards of confidentiality and academic integrity. Trust HOW.EDU.VN to provide expert guidance you can rely on.
11. FAQs About Finding Oxidation Numbers
Here are some frequently asked questions about finding oxidation numbers:
- What is the difference between oxidation number and oxidation state?
- Oxidation number and oxidation state are often used interchangeably. The oxidation number is a numerical representation of an atom’s oxidation state, while the oxidation state describes the degree of oxidation.
- What are the rules for assigning oxidation numbers?
- The rules for assigning oxidation numbers are outlined in Section 2 of this article.
- How do I find the oxidation number of an element in a compound?
- Follow the step-by-step guide in Section 3 of this article.
- What are some common mistakes to avoid when assigning oxidation numbers?
- Common mistakes to avoid are outlined in Section 5 of this article.
- What are the applications of oxidation numbers?
- The applications of oxidation numbers are outlined in Section 6 of this article.
- What are some advanced concepts related to oxidation numbers?
- Advanced concepts related to oxidation numbers are outlined in Section 7 of this article.
- How can HOW.EDU.VN help me master oxidation numbers?
- HOW.EDU.VN offers a range of resources to help you master oxidation numbers, as outlined in Section 8 of this article.
- Are there any exceptions to the oxidation number rules?
- Yes, there are exceptions to some of the rules, such as the oxidation number of oxygen in peroxides or hydrogen in metal hydrides.
- Can an element have a fractional oxidation number?
- Yes, in some compounds, the average oxidation number of an element may be a fraction.
- How do I assign oxidation numbers in organic compounds?
- Assigning oxidation numbers in organic compounds can be more challenging than in inorganic compounds due to the presence of covalent bonds and complex structures. However, the same basic principles apply.
12. Conclusion: Mastering Oxidation Numbers for Chemical Success
Finding oxidation numbers is a fundamental skill in chemistry. By understanding the rules, following the step-by-step guide, and avoiding common mistakes, you can master this essential concept. With the help of HOW.EDU.VN, you can take your chemistry knowledge to the next level and achieve academic success.
Don’t let complex chemical concepts hold you back. Contact HOW.EDU.VN today at 456 Expertise Plaza, Consult City, CA 90210, United States, or reach out via WhatsApp at +1 (310) 555-1212. Visit our website at how.edu.vn to connect with our team of expert PhDs and start your journey towards mastering chemistry. Our dedicated experts are ready to provide personalized solutions and reliable guidance tailored to your specific needs.